Diazoxide
General Information
Diazoxide Impurities and Diazoxide
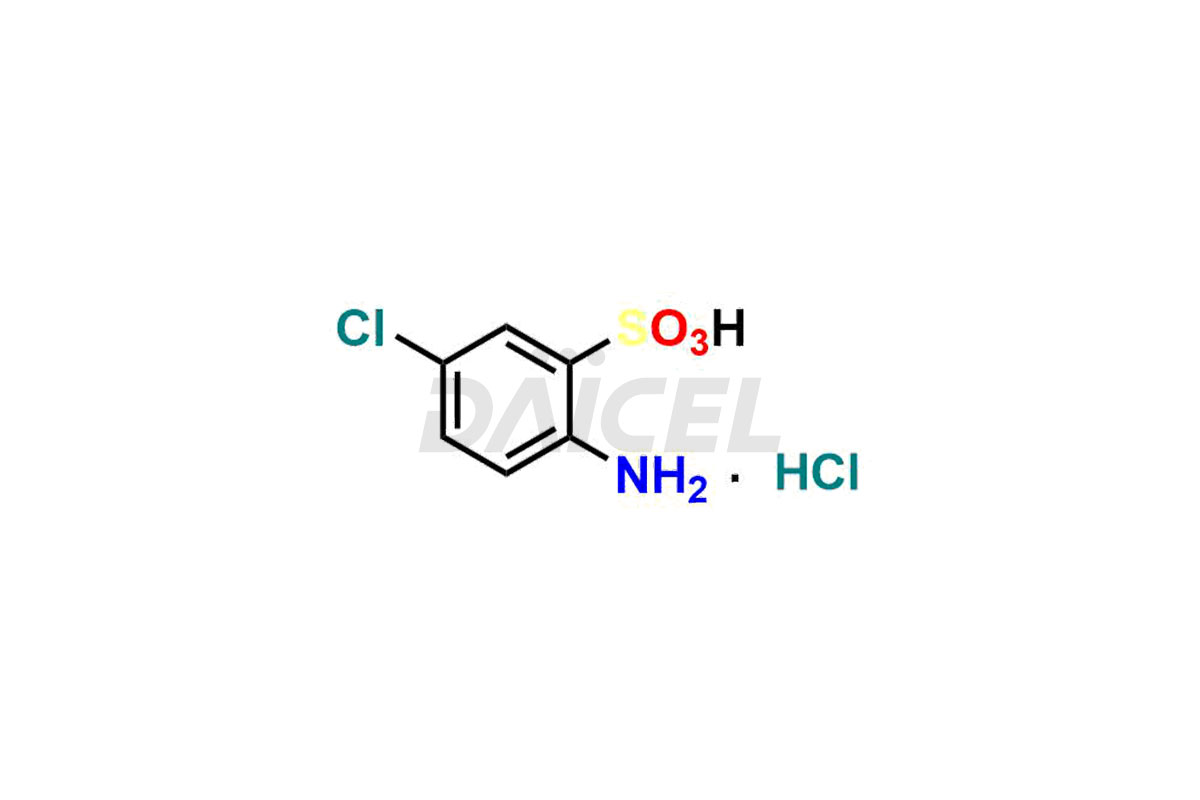
Daicel Pharma specializes in synthesizing high-quality impurities for Diazoxide, an active pharmaceutical ingredient. The impurity, 2-Amino-5-chlorobenzenesulfonic Acid hydrochloride, plays a vital role in assessing the purity, reliability, and safety of Diazoxide. Daicel Pharma also offers a customized synthesis of Diazoxide impurities to cater to client requirements, with worldwide delivery options available.
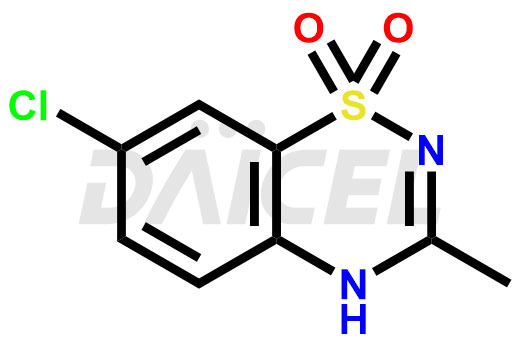
Diazoxide [CAS: 364-98-7], a benzothiadiazine derivative, acts on hypertension and hyperglycemia. It is a peripheral vasodilator, useful in hypertensive emergencies. Diazoxide offers a versatile pharmacological profile that addresses various therapeutic needs.
Diazoxide: Use and Commercial Availability
Diazoxide inhibits insulin secretion and promotes hepatic gluconeogenesis and glycogenolysis, limiting tissue glucose utilization. Consequently, it leads to elevated blood glucose levels (hyperglycemia). It also treats neonatal hyperinsulinism. It is available under brand names, including Hyperstat and Proglycem.
Diazoxide Structure and Mechanism of Action 
The chemical name of Diazoxide is 7-Chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide. Its chemical formula is C8H7ClN2O2S, and its molecular weight is approximately 230.67 g/mol.
Diazoxide interacts with ATP-sensitive potassium channels of pancreatic islet beta-cells and inhibits insulin release.
Diazoxide Impurities and Synthesis
Diazoxide impurities can form during manufacturing1 or due to degradation over time. They are of concern as they can affect the quality, efficacy, and safety of the final product. The impurities can impact the drug’s stability, pharmacokinetics, and potential adverse effects. Therefore, rigorous analysis and control of Diazoxide impurities are essential to meet regulatory requirements and ensure patient safety. It includes thorough characterization and quantification of impurities and implementing measures to minimize their formation during manufacturing and storage.
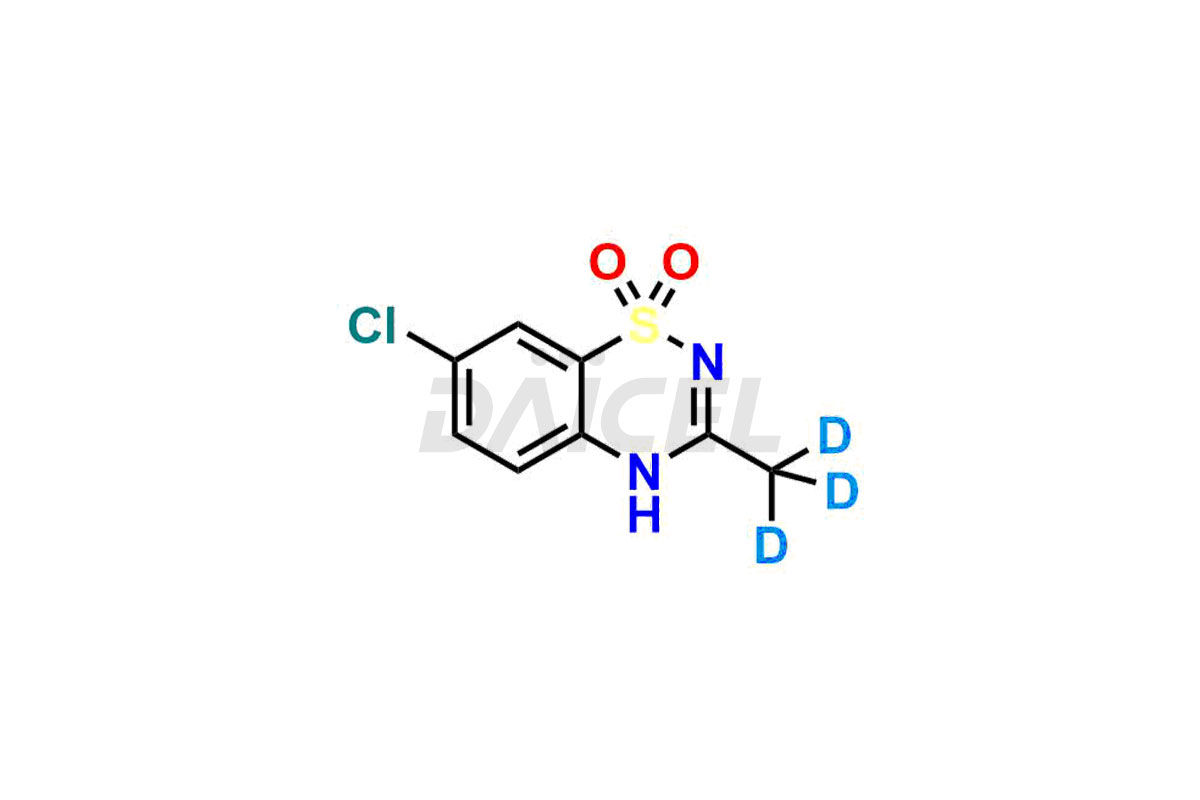
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Diazoxide impurity standards, including 2-Amino-5-chlorobenzenesulfonic Acid hydrochloride. They generate in an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Diazoxide impurities or degradation products, and labeled compounds, to evaluate the efficacy of generic Diazoxide. Also, Diazoxide-D3, a deuterium-labeled Diazoxide standard, is available for bio-analytical research, including BA/BE studies. Every delivery accompanies a complete characterization report.
References
FAQ's
References
- Topliss, John G.; Sperber, Nathan; Rubin, Alan A., Method for the treatment of hypertension, Schering Corp., US2986573A, May 30, 1961
- Sadee, Wolfgang; Segal, Jack; Finn, Claude, Diazoxide urine and plasma levels in humans by stable-isotope dilution-mass fragmentography, Journal of Pharmacokinetics and Biopharmaceutics, Volume: 1, Issue: 4, Pages: 295-305, 1973
Frequently Asked Questions
What is the significance of analyzing Diazoxide impurities?
Analyzing Diazoxide impurities helps ensure the drug's quality, safety, and efficacy. It helps determine the impurity level and its potential impact on the drug's effectiveness and stability.
How are the Diazoxide impurities synthesized?
Diazoxide impurities can be synthesized through chemical reactions involving Diazoxide or its intermediates. These reactions may occur during the drug synthetic process or under specific conditions for impurity formation.
Which solvent helps in the analysis of Diazoxide impurities?
Methanol is a solvent used in analyzing many impurities in Diazoxide.
What are the temperature conditions required to store Diazoxide impurities?
Diazoxide impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.