LOAD MORE
You're viewed 9 of 19 products
Daicel Pharma synthesizes high-quality Sacubitril impurities like (2R,4R)-Sacubitril, (2R,4S)-Sacubitril, (2S,4S)-Sacubitril, 3-Methyl Pyrrolidione (for Sacubitril), Sacubitril amino acid impurity, Sacubitril Ene impurity, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Sacubitril. Moreover, Daicel Pharma offers custom synthesis of Sacubitril impurities and delivers them globally.
Sacubitril [CAS: 149709-62-6] is a prodrug that inhibits neprilysin (NEP). It is a component of the drug containing Sacubitril/valsartan, a novel class of drugs called angiotensin receptor neprilysin inhibitors (ARNI). It reduces the risk of cardiovascular death in patients with chronic heart failure.
Sacubitril/valsartan is for patients with chronic heart failure with reduced ejection fraction (HFrEF) who continue to experience symptoms despite receiving optimal medical treatment with ACE inhibitors, ARBs, beta-blockers, and MRA. It is a substitute for ACE inhibitors or other ARBs to decrease the risk of hospitalization and death due to cardiovascular events. It is also used with other heart failure treatments. Sacubitril/valsartan is available under the trade name Entresto.
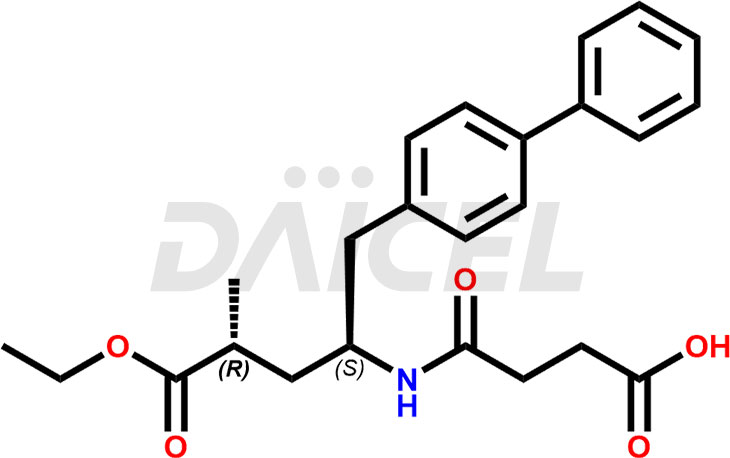
The chemical name of Sacubitril is (2R,4S)-5-(Biphenyl-4-yl)-4-[(3-carboxypropionyl)amino]-2-methylpentanoic acid ethyl ester. Its chemical formula is C24H29NO5, and its molecular weight is approximately 411.5 g/mol.
Sacubitril inhibits neprilysin, combines with angiotensin receptor blocker Valsartan and helps in vasodilation and natriuresis. It reduces the risk of heart failure in patients having chronic heart failure.
During the production1 of Sacubitril, impurities form that may be harmful to patients. These impurities are due to factors such as reaction conditions, the quality of starting materials, and the presence of other reaction intermediates. Purification methods, such as chromatography and crystallization, are used to remove these impurities and ensure the purity and safety of the final product.
Daicel provides a Certificate of Analysis (CoA) for Sacubitril impurity standards, including (2R,4R)-Sacubitril, (2R,4S)-Sacubitril, (2S,4S)-Sacubitril, 3-Methyl Pyrrolidione (for Sacubitril), Sacubitril amino acid impurity, Sacubitril Ene impurity, and more. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Sacubitril impurity or degradation product and offer labeled compounds to quantify the efficacy of Sacubitril. Daicel offers Sacubitril – D4 and Sacubitril metabolite LBQ 657 – D4 / Sacubitrilat/D4 (New), deuterium-labeled Sacubitril standards, used in bio-analytical research, such as BA/BE studies. We give a complete characterization report on delivery.
The challenges associated with detecting and quantifying impurities in Sacubitril include its low level, the complex manufacturing process, and the usage of sensitive and specific analytical techniques. The variability of impurities between different batches of the drug can also be a challenge.
The Impurities in Sacubitril affect its stability and shelf life by promoting degradation reactions that reduce the drug’s potency over time. A few impurities also affect the physical properties of the drug, such as its solubility and crystallinity.
Methanol or Acetonitrile is the solvent used in analyzing many impurities in Sacubitril.
Sacubitril impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.