LOAD MORE
You're viewed 9 of 30 products
Daicel Pharma synthesizes high-quality Octreotide impurities, (N-O) acyl isomer of Octreotide, Ac-Octreotide, D-allo-Thr(6)-Octreotide, D-Cys(2)-Octreotide, D-Phe(3)-Octreotide, Linear Octreotide, Octreotide Acid and more, that help in the quality, stability, and biological safety analysis of Octreotide. We also offer custom synthesis of Octreotide impurities and supply worldwide.
Octreotide [CAS: 83150-76-9] is a synthetic peptide similar in structure to somatostatin, a natural hormone produced by the hypothalamus. Octreotide is a longer-acting and more potent version of somatostatin, and it is used in medical treatment to inhibit the secretion of certain hormones and neurotransmitters.
Octreotide is an FDA-approved medicine primarily used to treat acromegaly, thyrotrophinomas, and carcinoid syndrome. It is also effective in reducing stool or fistula output in patients with high-output secretory diarrhea and treating various conditions such as hyperinsulinemia-induced neonatal hypoglycemia, insulin-dependent diabetes mellitus, reactive pancreatitis, dumping syndrome, and postprandial hypotension. Although not approved by the FDA, Octreotide has also been used off-label to treat refractory or persistent diarrhea associated with chemotherapy, graft-versus-host disease, and AIDS-associated diarrhea caused by cryptosporidiosis. It has shown potential in managing metastatic gastroenteropancreatic neuroendocrine tumors, advanced thymoma, preventing carcinoid crisis, hepatorenal syndrome, hypoglycemia due to sulfonylurea, congenital hyperinsulinism, ectopic Cushing syndrome, hypothalamic obesity, Zollinger-Ellison syndrome, dumping syndrome after gastrectomy, and small intestinal fistulas.
Octreotide can be administered subcutaneously or intravenously. The long-acting release form has a lower bioavailability of about 60% when compared to the subcutaneous form. It is available under various brand names, including Sandostatin, Sanostatine, Longastatin, Mycapssa, and Sandostatin LAR.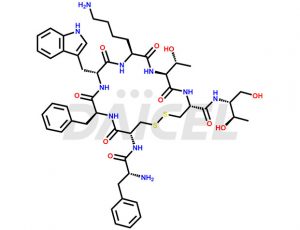
The chemical formula of Octreotide is C49H66N10O10S2, and its molecular weight is 1019.24 g/mol.
Octreotide exerts pharmacologic actions like somatostatin but is a more potent inhibitor of glucagon, GH, and insulin than somatostatin. In addition, it suppresses the Luteinizing hormone (LH) response to the gonadotropin-releasing hormone (GnRH). Further, it inhibits the release of serotonin, gastrin, secretin, pancreatic polypeptide, motilin, and vasoactive intestinal peptide.
Octreotide is synthesized1 using solid-phase peptide synthesis. During the manufacturing process, various impurities may form like oxidation products, deamidated and truncated peptides, and side products. These impurities can affect the quality and stability of the final product and may also have potential toxicity concerns. Therefore, quality control measures are put in place during the manufacturing process to ensure that the level of impurities is within acceptable limits.
At Daicel, we provide a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for more than twenty Octreotide impurity standards including (N-O) acyl isomer of Octreotide, Ac-Octreotide, D-allo-Thr(6)-Octreotide, D-Cys(2)-Octreotide, D-Phe(3)-Octreotide, Linear Octreotide, Octreotide Acid and more, along with complete characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Upon request, we provide 13C-DEPT and CHN. Also, we provide a complete characterization report upon delivery. Daicel offers Octreotide D8 (Labelled), which is a deuterium-labeled standard of Octreotide in bioanalytical research and BA/BE studies with isotope data in CoA.
Octreotide impurities are removed through a combination of purification techniques, such as chromatography, crystallization, and filtration.
Octreotide impurities are identified using analytical techniques such as high-performance liquid chromatography (HPLC), and liquid chromatography-mass spectrometry (LC-MS).
The synthesis of Octreotide impurities involves the use of various chemical reactions, such as peptide coupling, esterification, and protection/deprotection steps.
Impurities can have a significant impact on the safety, efficacy, and stability of Octreotide. They can affect the drug's pharmacological properties, increase the risk of adverse reactions, and reduce the shelf life of the drug.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.