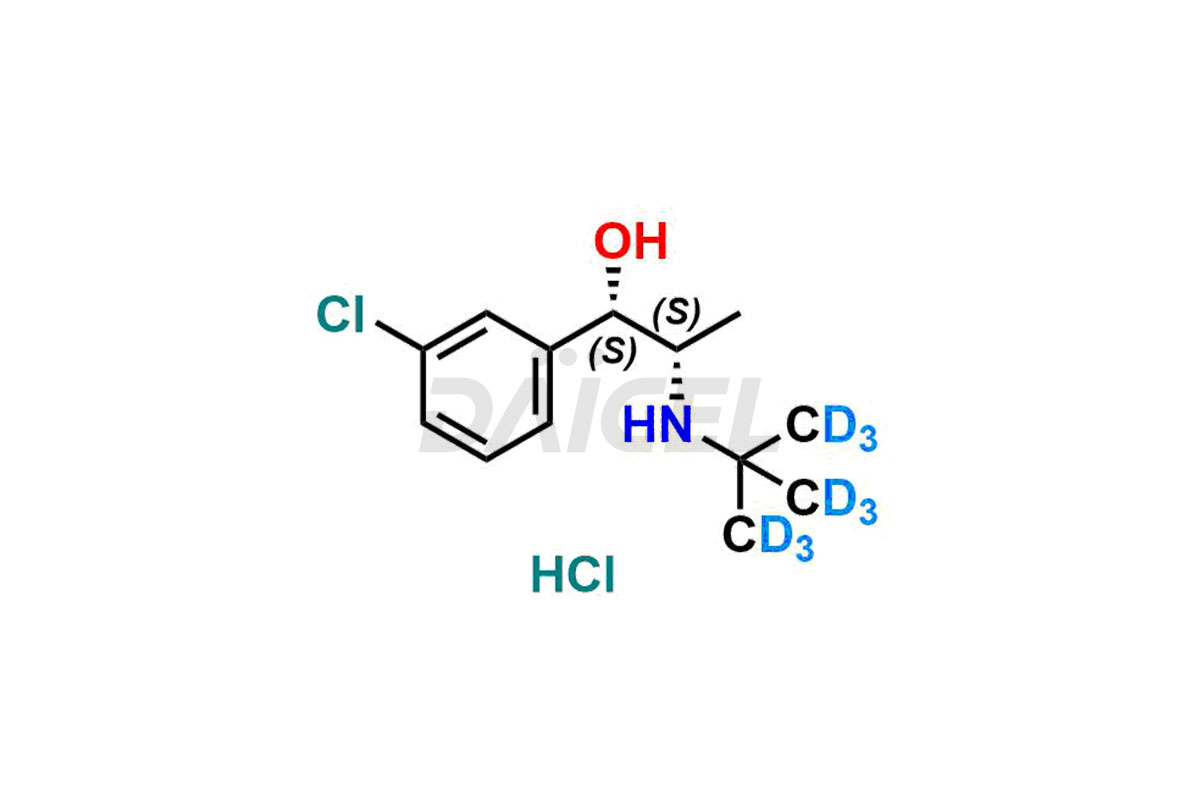
Threo-Dihydro Bupropion.HCl – D9
- CAT Number DCTI-A-099
- CAS Number 1217815-08-1
- Molecular Formula C13H12D9Cl2NO (HCl Salt) C13H11D9ClNO (Free base)
- Molecular Weight 287.27 (HCl Salt) 250.81 (Free base)
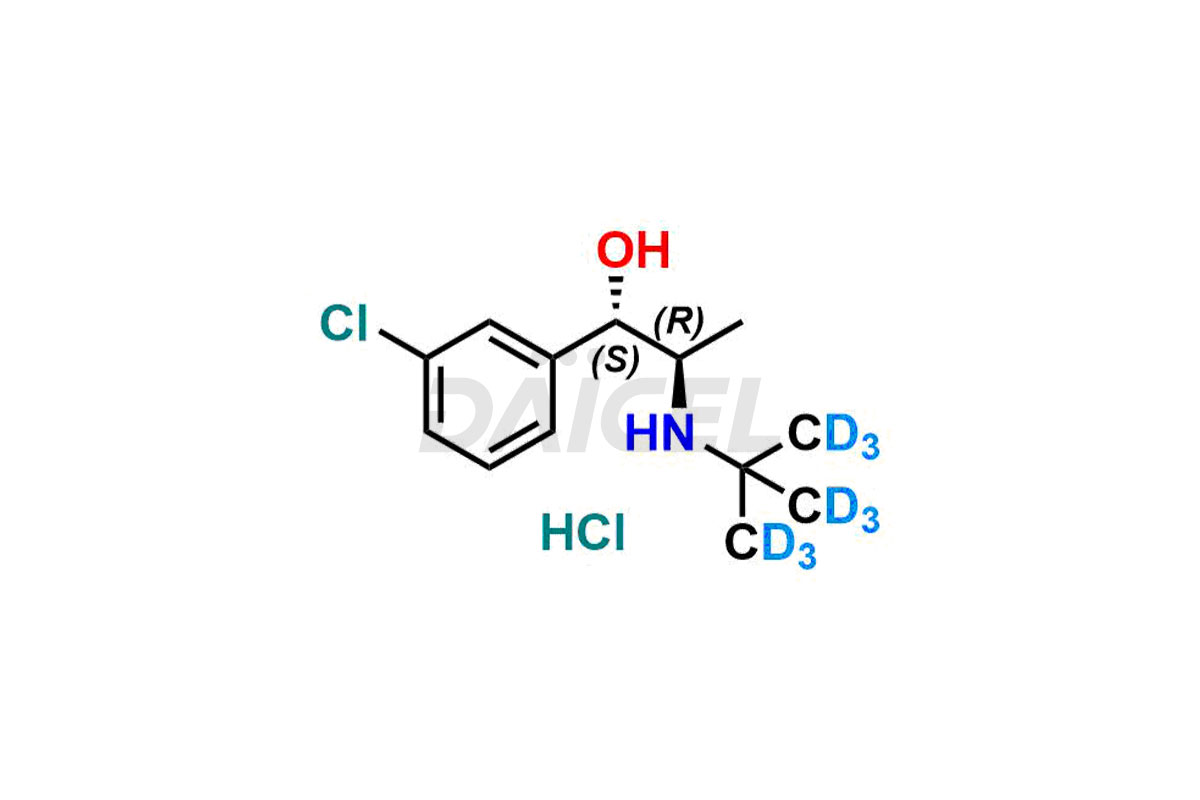
Erythro-Dihydro Bupropion.HCl – D9
- CAT Number DCTI-A-100
- CAS Number 1217684-77-9(Freebase)
- Molecular Formula C13H12D9Cl2NO (HCl Salt) C13H11D9ClNO (Free base)
- Molecular Weight 287.27 (HCl Salt) 250.81 (Free base)
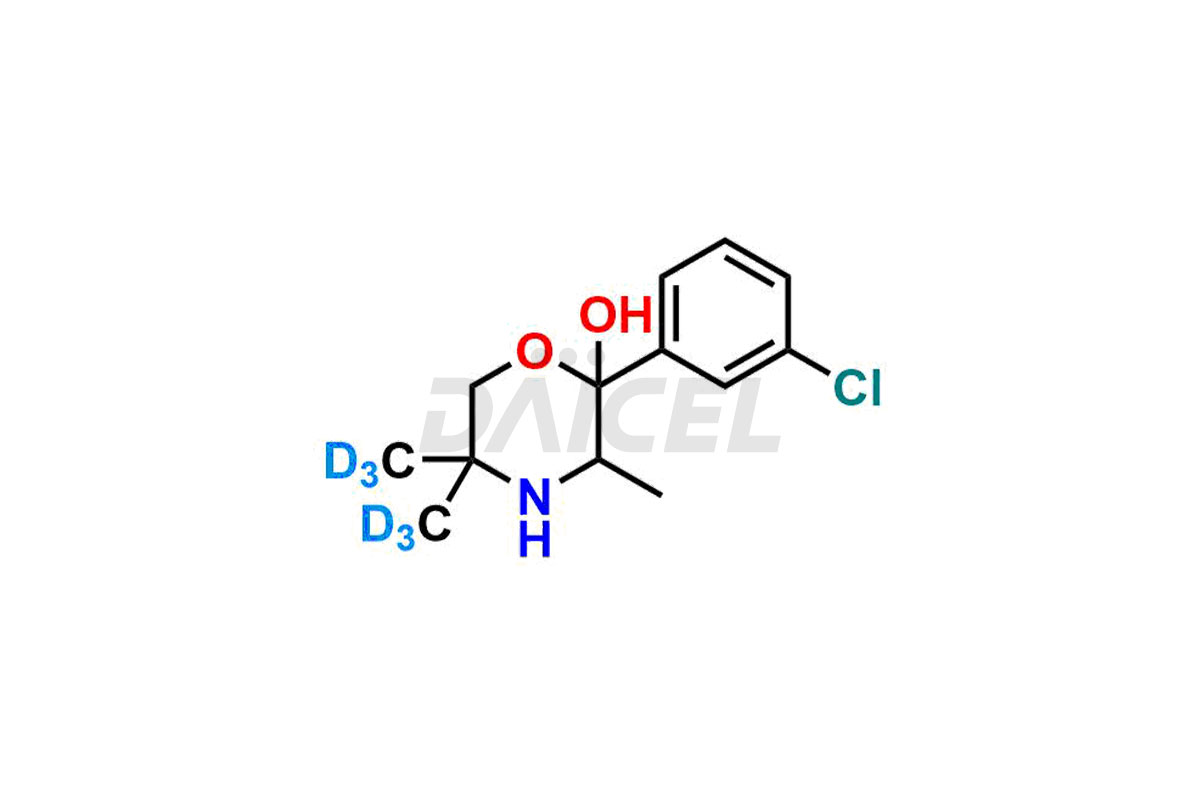
Hydroxy Bupropion-D6
- CAT Number DCTI-A-096
- CAS Number 1216893-18-3
- Molecular Formula C13H12D6ClNO2
- Molecular Weight 261.78
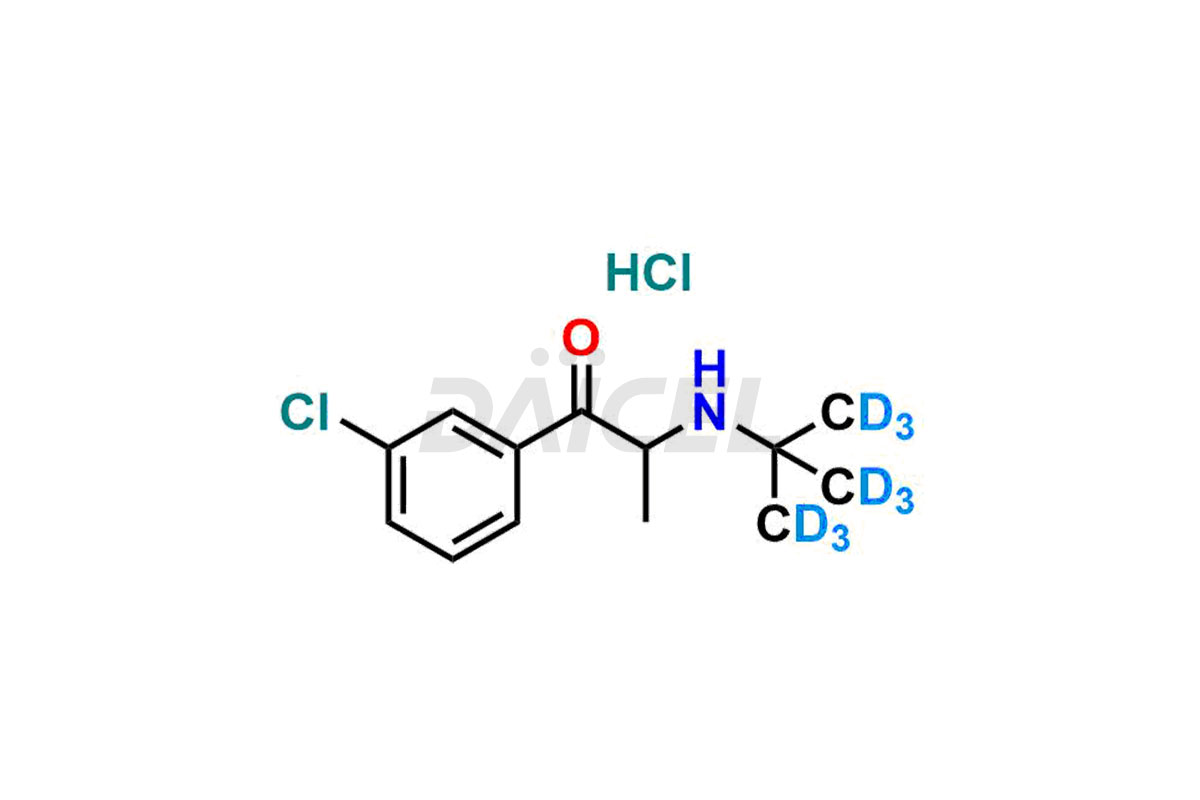
Bupropion Hydrochloride-D9
- CAT Number DCTI-A-075
- CAS Number 1189725-26-5
- Molecular Formula C13H10D9Cl2NO (HCl Salt) C13H9D9ClNO (Free base)
- Molecular Weight 285.26 (HCl Salt) 248.80 (Free base)