Adenosine
General Information
Adenosine Impurities and Adenosine
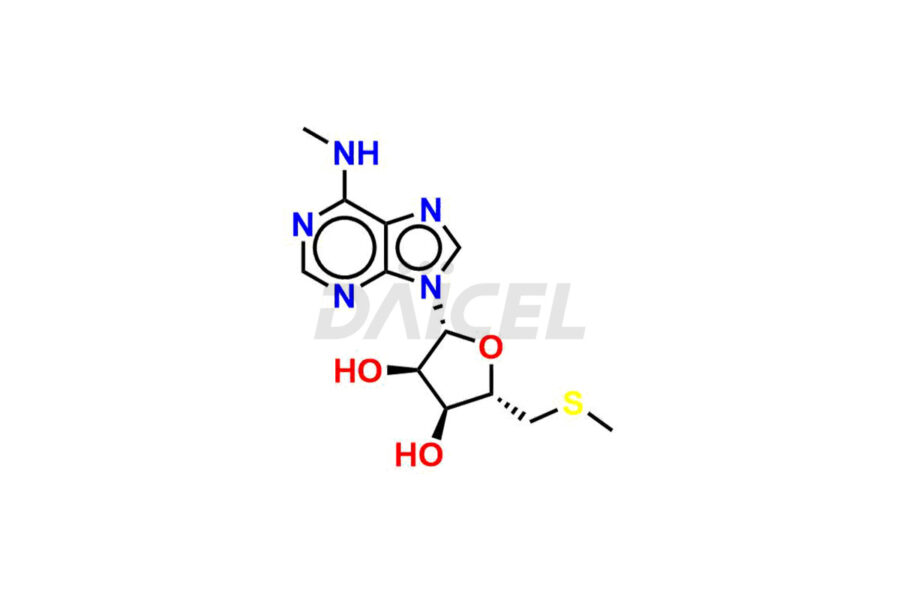
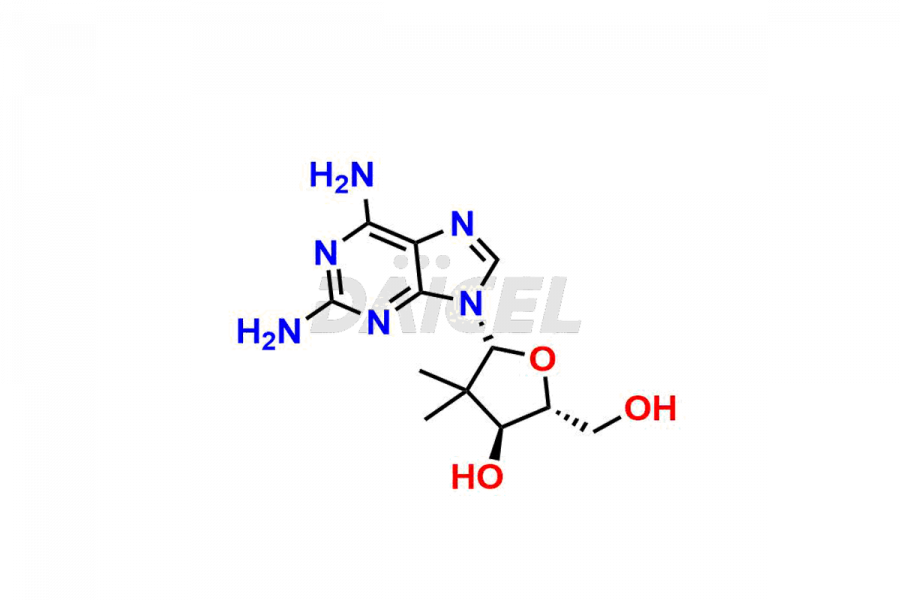
Daicel Pharma offers high-quality Adenosine impurities, (2R, 3R, 4S, 5S)-2-(6-(methylamino)-9H-purin-9-yl)-5-((methylthio)methyl)tetrahydrofuran-3,4-diol, and 2-Amino-2′-deoxy-2′,2′-dimethyl-Adenosine, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Adenosine. Moreover, Daicel Pharma offers custom synthesis of Adenosine impurities and delivers them globally.
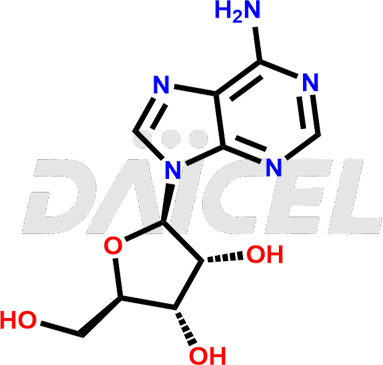
Adenosine [CAS: 58-61-7] is a ribonucleoside that binds Adenine to ribose. It has several functions, including vasodilation, antiarrhythmic properties, and analgesic effects. Adenosine has phosphorylated forms and helps in various cellular processes, including cell energy transfer, signal transduction, and RNA synthesis.
Adenosine: Use and Commercial Availability
Adenosine has diagnostic and therapeutic applications, working through purinergic Adenosine receptors found in the body. Its vasodilatory effects act as a diagnostic agent in myocardial perfusion stress imaging. Its antiarrhythmic properties act as a therapeutic agent in supraventricular tachycardia. Adenosine helps as an adjunct to thallium-201 in myocardial perfusion scintigraphy for patients unable to exercise adequately and to convert the sinus rhythm of paroxysmal supraventricular tachycardia. The drug is available under the brand names Adenocard and Adenoscan.
Adenosine Structure and Mechanism of Action
The chemical name of Adenosine is 9-β-D-Ribofuranosyl-9H-purin-6-amine. Its chemical formula is C10H13N5O4, and its molecular weight is approximately 267.24 g/mol.
Adenosine causes vasodilation in many vessels and helps in the metabolic control of organ perfusion. However, it causes vasoconstriction in renal afferent arterioles and hepatic veins.
Adenosine Impurities and Synthesis
Adenosine impurities occur in various categories such as organic, inorganic, and residual solvents. They may occur during synthesis1, storage, or degradation of the active pharmaceutical ingredient (API). It is essential to identify, monitor, and control these impurities to ensure the safety and effectiveness of the drug product.
Daicel provides a Certificate of Analysis (CoA) for Adenosine impurity standards, (2R, 3R, 4S, 5S)-2-(6-(methylamino)-9H-purin-9-yl)-5-((methylthio)methyl)tetrahydrofuran-3,4-diol and 2-Amino-2′-deoxy-2′,2′-methyl-Adenosine. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data2, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Adenosine impurity or degradation product. We give a complete characterization report on delivery.
References
FAQ's
References
- Asai, Motoji; Hieda, Harukiyo; Shimizu, Bunji, Synthetic nucleotides. IV. Preparation of L-β-ribonucleosides and L-β-ribonucleotides, Chemical & Pharmaceutical Bulletin, Volume: 15, Issue: 12, Pages: 1863-70, 1967
- Rossi, Luigi; Rossi, Luigi, Paper-chromatographic separation of adenine metabolites: purine bases, nucleosides, and nucleotides, Journal of Chromatography, Volume: 30, Issue: 1, Pages: 278-83, 1967
Frequently Asked Questions
How are Adenosine impurities detected and quantified?
Impurities in Adenosine can be detected and quantified using various analytical techniques, including high-performance liquid chromatography (HPLC) and Liquid chromatography-mass spectrometry (LC-MS).
Do Adenosine impurities affect the pharmacological properties of the drug?
The impurities in Adenosine affect the drug’s pharmacological properties, such as efficacy, safety, and bioavailability. It can lead to unpredictable harmful effects on patients.
Which solvent helps in the analysis of Adenosine impurities?
Methanol is a solvent used for analyzing many impurities in Adenosine.
What are the temperature conditions required to store Adenosine impurities?
Adenosine impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.