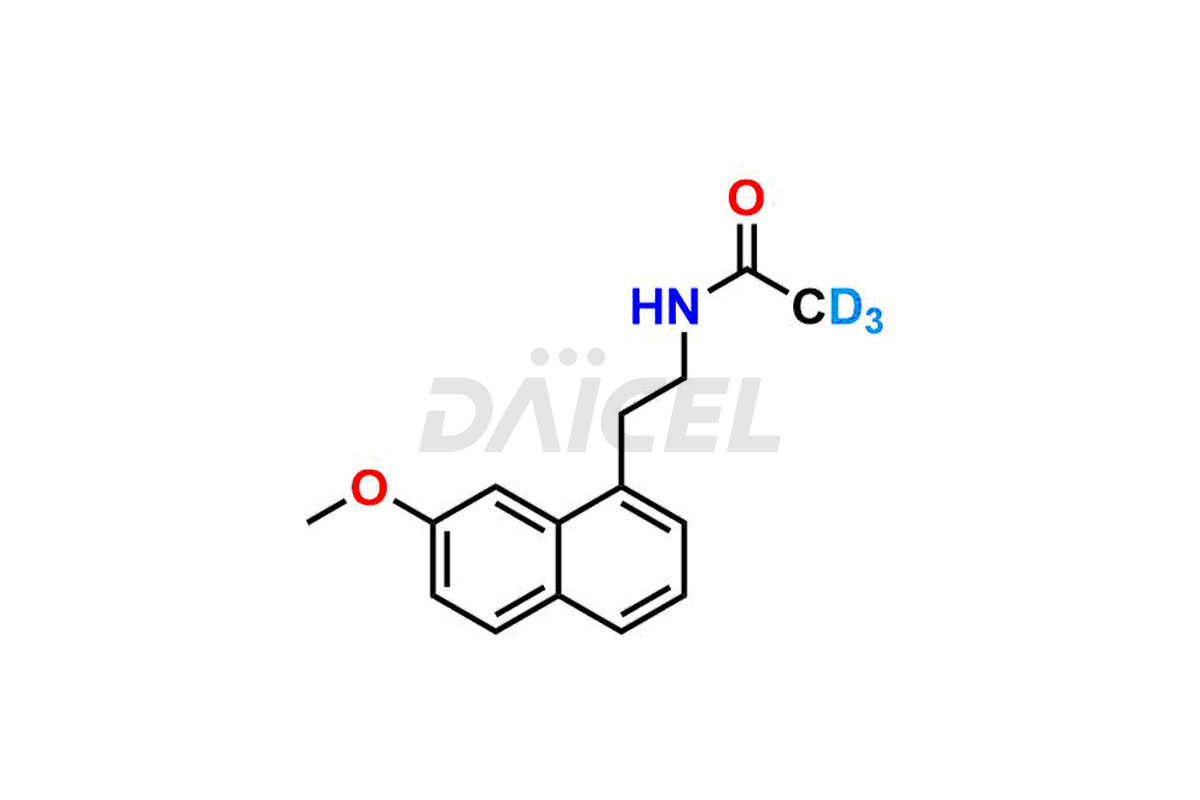
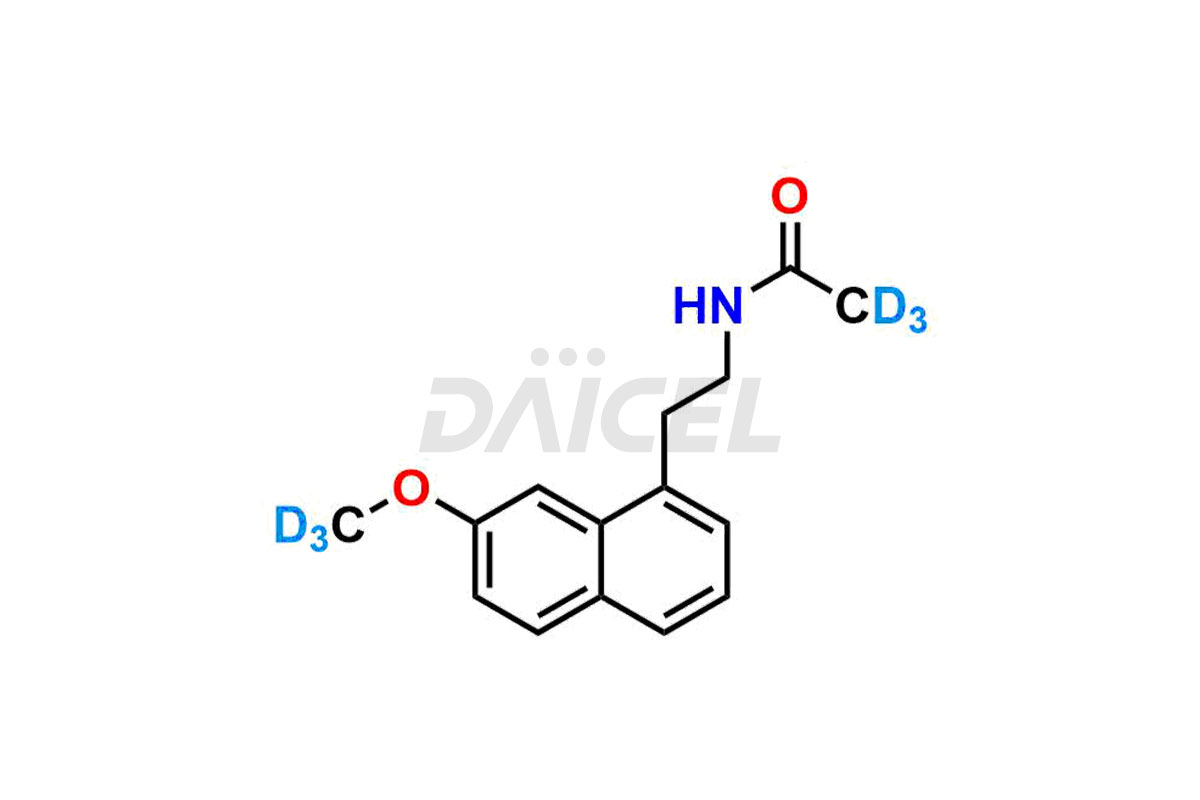
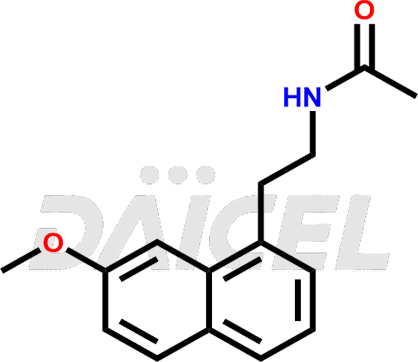
Agomelatine
General Information
Agomelatine Impurities and Agomelatine
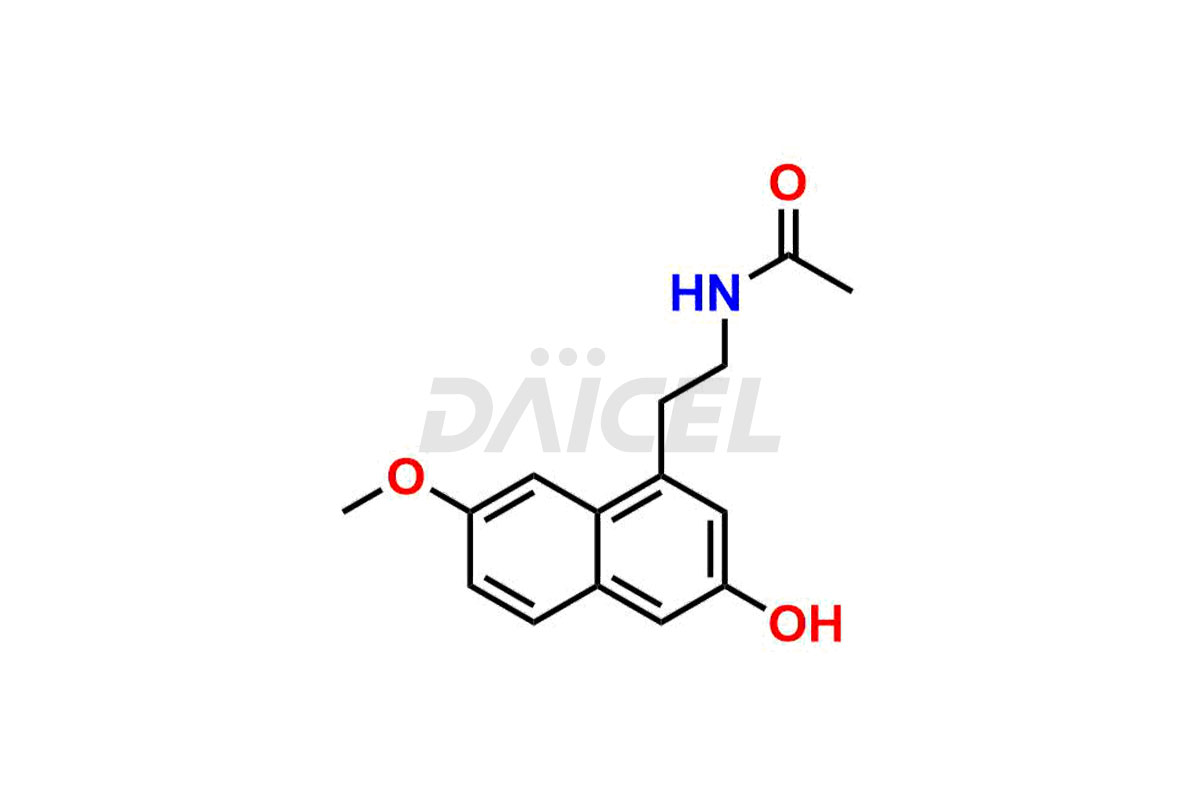
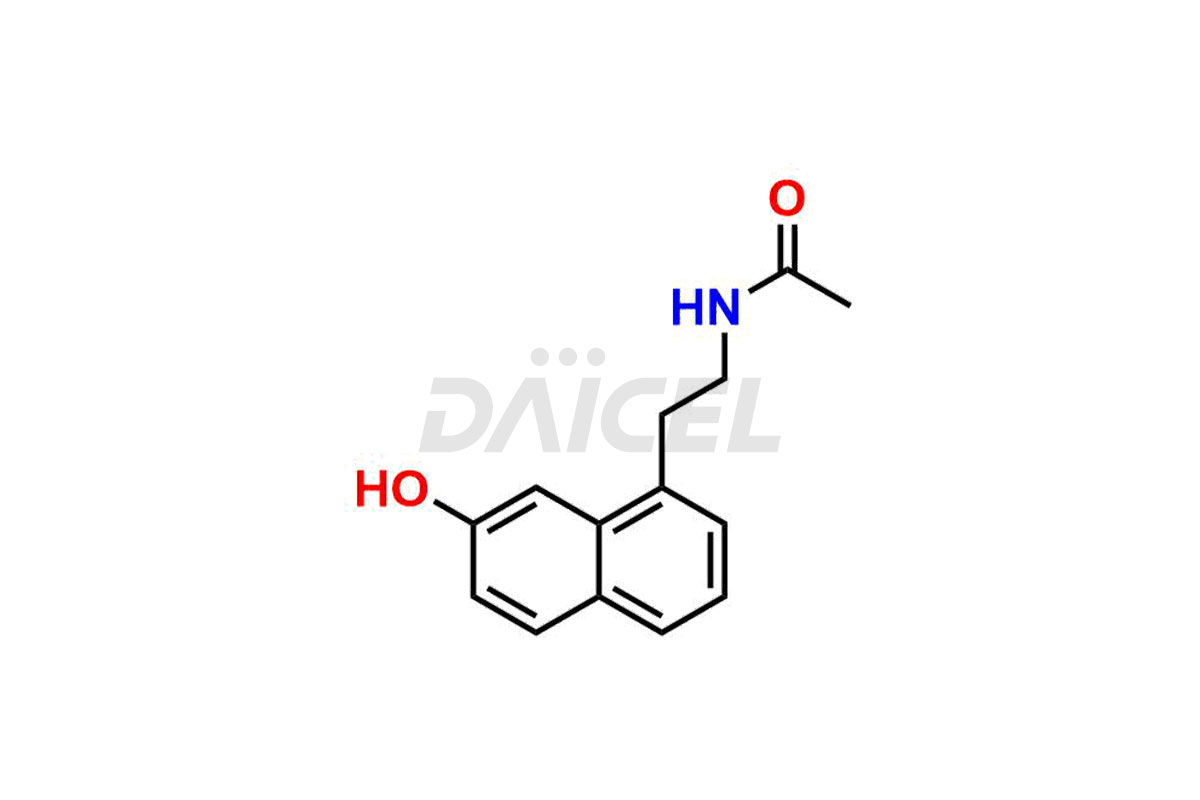
Daicel Pharma synthesizes high-quality Agomelatine impurities like 3-Hydroxy Agomelatine and 7-Desmethyl Agomelatine, which are crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient Agomelatine. Moreover, Daicel Pharma offers custom synthesis of Agomelatine impurities and delivers them globally.
Agomelatine [CAS: 138112-76-2] is an antidepressant, an acetamide naphthalene analog of melatonin. It helps in antidepressant therapy, including Major Depressive Disorder (MDD).
Agomelatine: Use and Commercial Availability
Agomelatine is not US FDA-approved for marketing in the US. However, it is an approved medicine in Europe for treating depression. It treats other diseases such as seasonal affective disorder, sleep disorders, cardiovascular pathologies, pathologies of the digestive system, insomnia, fatigue due to jet lag, appetite disorders, and obesity. Agomelatine has dual agonist activity at melatonin MT1 and MT2 receptors and antagonist activity at serotonergic 5-HT2C receptors. The medicine is available under the brand names Valdoxan and Thymanax.
Agomelatine Structure and Mechanism of Action
The chemical name of Agomelatine is N-[2-(7-Methoxy-1-naphthalenyl)ethyl]acetamide. Its chemical formula is C15H17NO2, and its molecular weight is approximately 243.30 g/mol.
Agomelatine, a melatonergic agonist, mimics the natural rhythm of melatonin release. It acts as a serotonin antagonist at the 5-HT2C receptor, enhancing dopamine and noradrenaline levels in the frontal cortex and producing neurogenesis effects.
Agomelatine Impurities and Synthesis
Agomelatine has limited information on its impurities. However, studies have reported the presence of degradation products, process impurities, and solvents as potential impurities in Agomelatine. They form due to various factors, including degradation under stress conditions, incomplete reactions during synthesis1,2, and impurities in raw materials. It is necessary to control these impurities to ensure Agomelatine quality, safety, and efficacy through appropriate analytical techniques, process optimization, and adherence to regulatory guidelines.
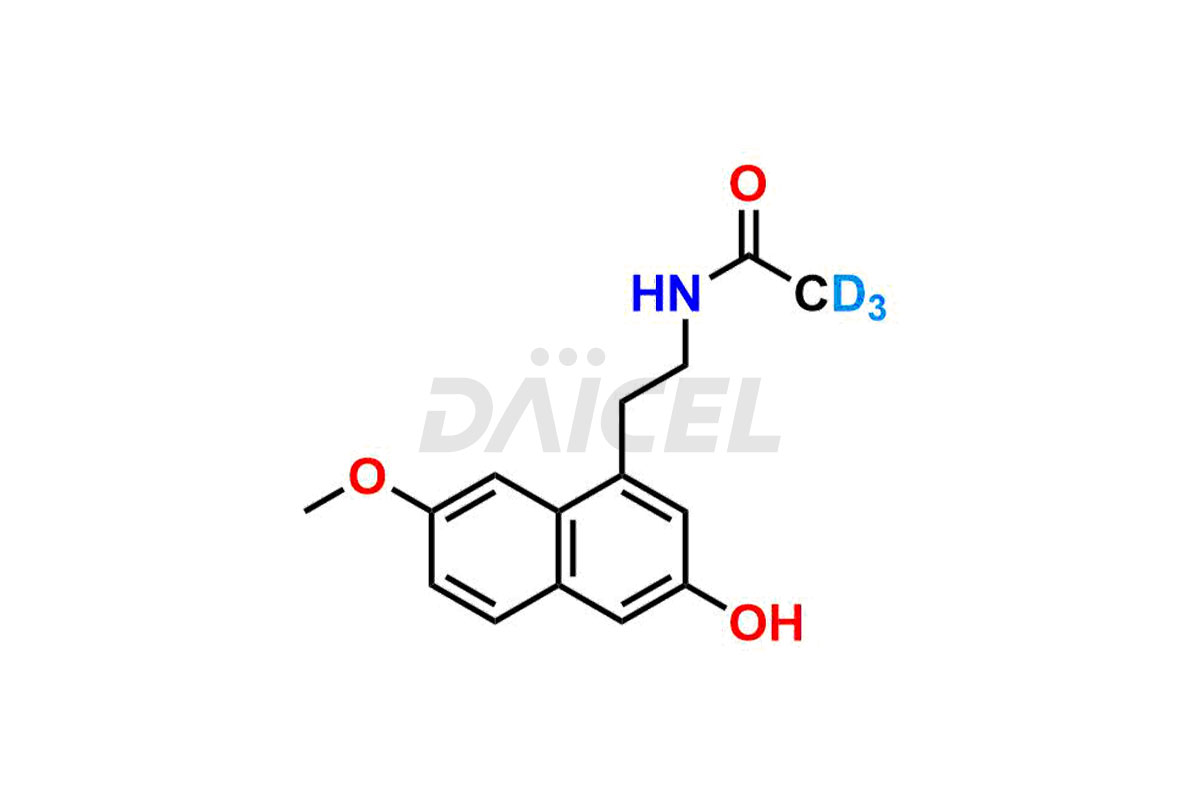
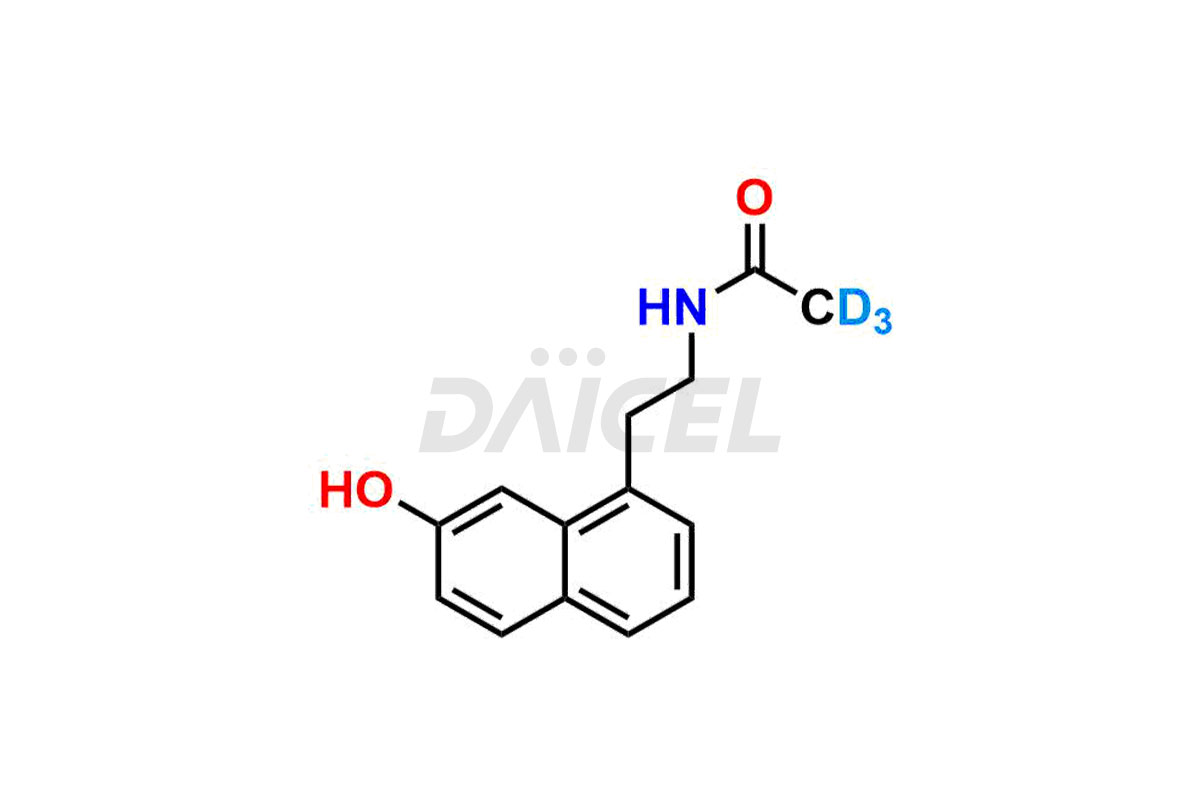
Daicel provides a Certificate of Analysis (CoA) for Agomelatine impurity standards, including 3-Hydroxy Agomelatine and 7-Desmethyl Agomelatine. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Agomelatine impurity or degradation product and offer labeled compounds to quantify the efficacy of generic Agomelatine. Daicel offers 3-Hydroxy Agomelatine – D3, 7-Desmethyl Agomelatine – D3, Agomelatine – D3, and Agomelatine – D6, deuterium-labeled Agomelatine compounds used in bio-analytical research such as BA/BE studies. We give a complete characterization report on delivery.
References
FAQ's
References
- Andrieux, Jean; Houssin, Raymond; Yous, Said; Guardiola, Beatrice; Lesieur, Daniel, Naphthalene Derivatives, Procedure For Their Preparation And Pharmaceutical Compositions Containing Them, EP447285B1, September, 18, 1991
- Markl, Christian; Zlotos, Darius P., A novel synthesis of the antidepressant agomelatine, Synthesis, Issue: 1, Pages: 79-82, 2011
- Patil, Satish R.; Nerurkar, Ketan K.; Kalamkar, Ashok M.; Pukale, Vishwas; Mangaonkar, Kiran V.; Pingale, Satish G., Validated LC-MS/MS method for quantification of agomelatine in human plasma and its application in a pharmacokinetic study, Journal of Mass Spectrometry, Volume: 47, Issue: 1, Pages: 23-28, 2012
Frequently Asked Questions
What is the role of impurity profiling in Agomelatine?
Impurity profiling helps identify and quantify impurities in Agomelatine and develops appropriate control strategies to ensure the drug's quality, safety, and efficacy.
Do impurities in Agomelatine affect the stability of the drug?
Impurities in Agomelatine can affect the stability of the drug by accelerating its degradation or reacting with the drug substance or excipients.
Which solvent helps in the analysis of Agomelatine impurities?
Methanol is a solvent used in analyzing many impurities in Agomelatine.
What are the temperature conditions required to store Agomelatine impurities?
Agomelatine impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.