LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma is a reliable source for synthesizing high-quality Alcaftadine impurities, specifically Alcaftadine Metabolite-7 (R087010), Alcaftadine Metabolite-3 (R087314), Alcaftadine N-demethylated, etc. These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Alcaftadine. Daicel Pharma also offers a custom synthesis of Alcaftadine impurities, which can be shipped worldwide to meet customers’ unique requirements.
Alcaftadine [CAS: 147084-10-4] is an imidazobenzazepine derivative treating allergic conjunctivitis. It acts as an antagonist of the H1 histamine receptor that prevents itching caused by allergic conjunctivitis.
Alcaftadine, known under Lastacaft, is an ophthalmic medication that acts as a dual-acting H1-antihistamine and mast cell stabilizer. Alcaftadine is an effective medication for preventing ocular itching caused by allergic conjunctivitis. Further, it has been shown to decrease chemotaxis and inhibits eosinophil activation.
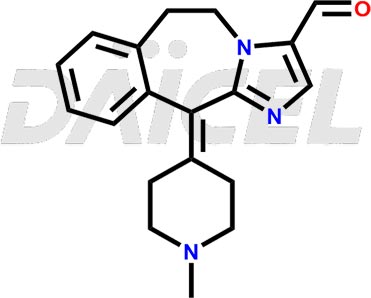
The chemical name of Alcaftadine is 6,11-Dihydro-11-(1-methyl-4-piperidinylidene)-5H-imidazo[2,1-b][3]benzazepine-3-carboxaldehyde. Its chemical formula is C19H21N3O, and its molecular weight is approximately 307.4 g/mol.
Alcaftadine inhibits histamine release from the mast cells.
Alcaftadine is an antihistamine drug that can be affected by impurities during their manufacturing. These impurities can arise from the starting materials, intermediates, and reagents used in the synthesis1 of the drug. They can reduce the drug’s effectiveness and pose risks to patient safety if not controlled. Therefore, it is essential to closely monitor and control the impurity levels in Alcaftadine to ensure the drug’s quality, efficacy, and safety.
Daicel Pharma provides a Certificate of Analysis (CoA) for Alcaftadine impurity standards, which includes Alcaftadine Metabolite-7 (R087010), Alcaftadine Metabolite-3 (R087314), Alcaftadine N-demethylated, etc. The CoA is from an analytical facility that adheres to current Good Manufacturing Practices (cGMP) and includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can also generate unknown Alcaftadine impurities or degradation products and provide labeled compounds to assess the effectiveness of Alcaftadine. Further, Daicel Pharma offers Alcaftadine 3-Carboxylic Acid D3 and Alcaftadine-D3, deuterium-labeled Alcaftadine compounds used in bio-analytical research, such as BA/BE studies. A complete characterization report is part of each delivery.
Impurities in Alcaftadine can impact the drug's stability and shelf life, potentially reducing its effectiveness over time. Therefore, controlling impurity levels is critical to ensure the drug's quality and shelf life.
Manufacturers determine the acceptable levels of impurities in Alcaftadine based on regulatory guidelines and scientific data. They must demonstrate that thery do not exceed safe limits and the drug remains safe and effective.
Impurities in Alcaftadine can change over time or in different storage conditions. For example, they may degrade or form new impurities under exposure to light, heat, moisture, or other factors. Therefore, manufacturers must test and monitor the drug under different storage conditions and use appropriate packaging and labeling to prevent degradation.
Alcaftadine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.