Amiodarone
General Information
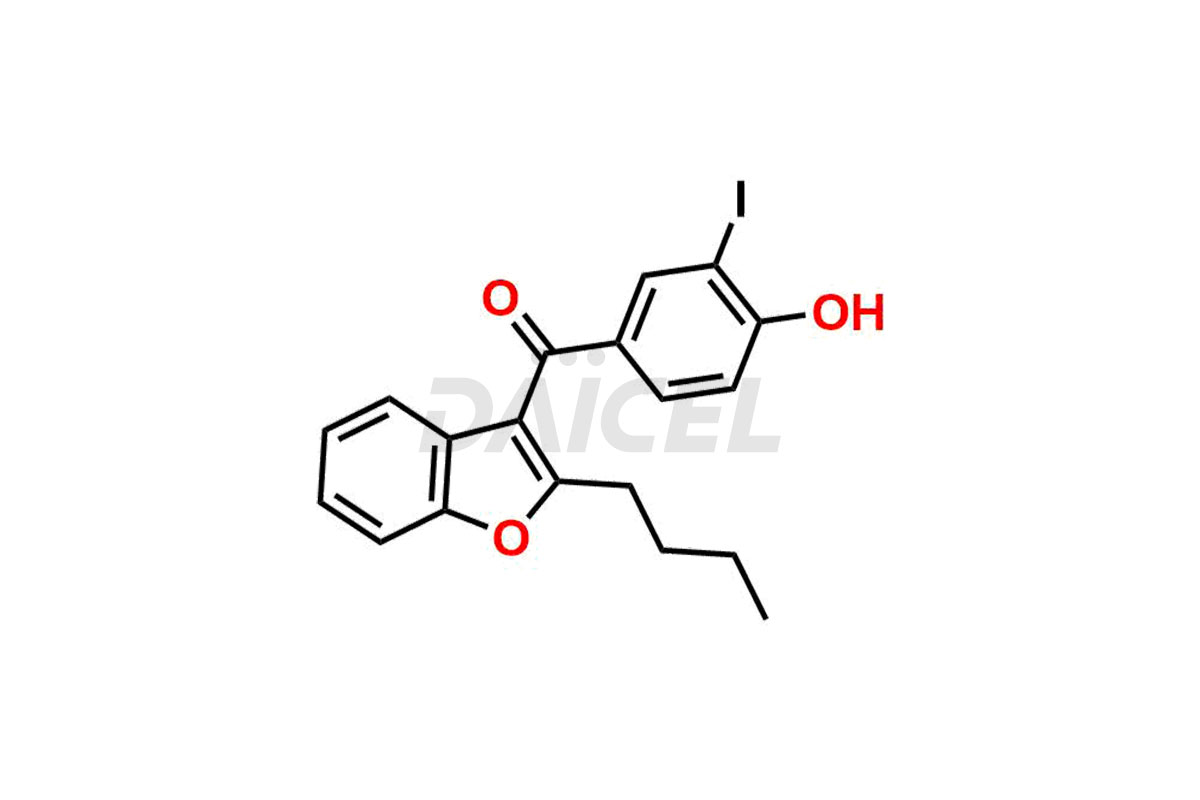
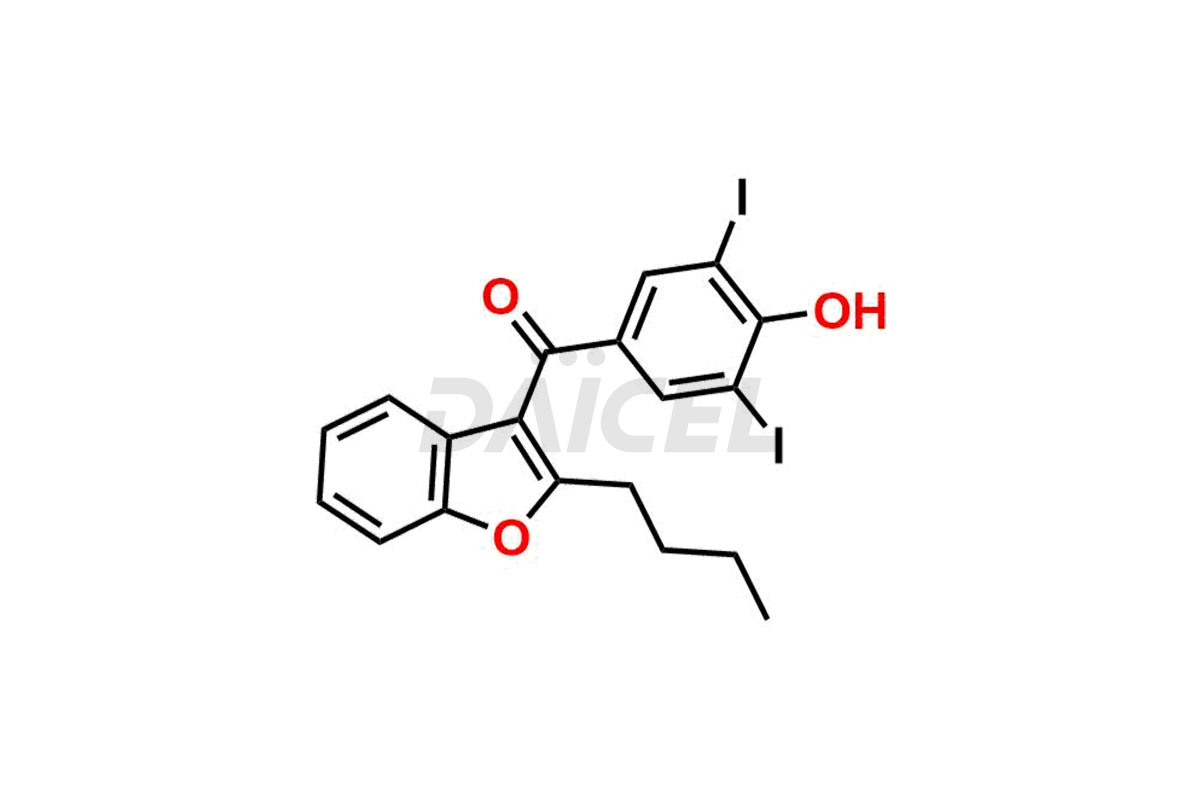
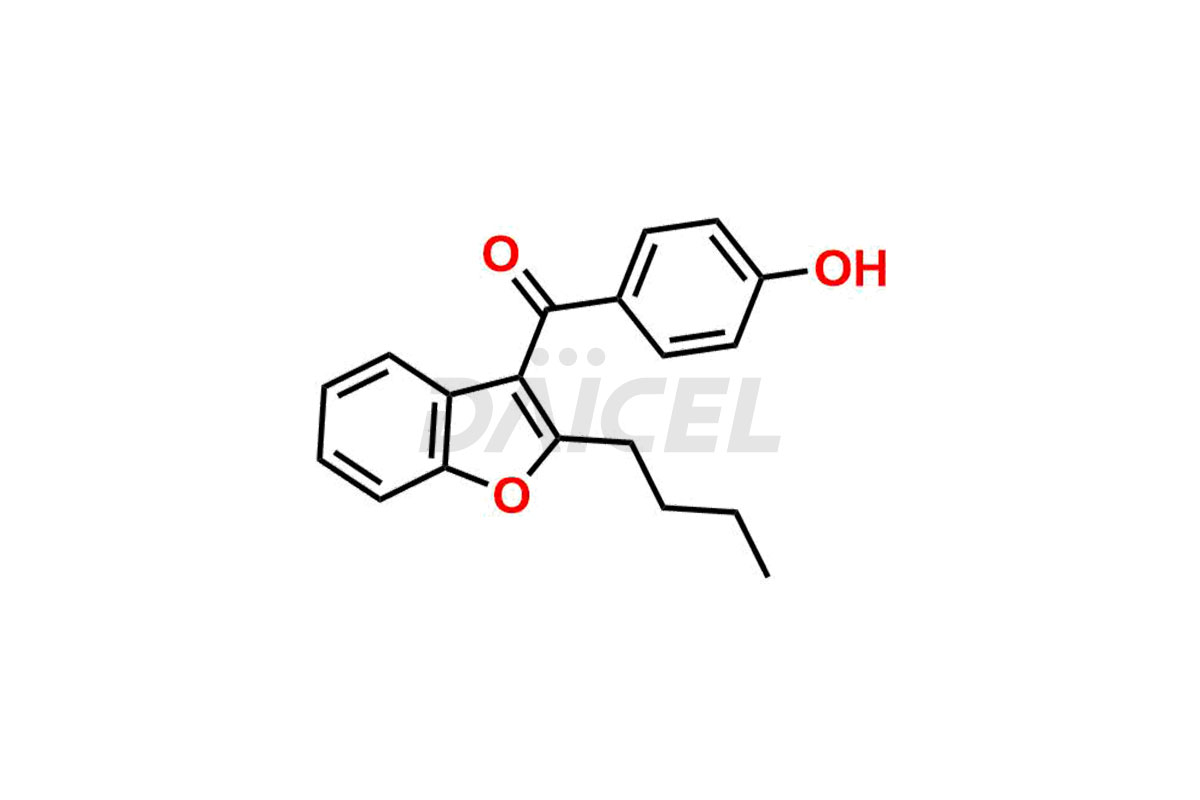
Amiodarone Impurities and Amiodarone
Daicel Pharma synthesizes high-quality Amiodarone impurities, including Amiodarone EP Impurity F, Amiodarone Impurity D, and Amiodarone Impurity E. These impurities are essential for evaluating the quality, stability, and safety of Amiodarone, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Amiodarone impurities delivered globally to meet the specific needs of our customers.
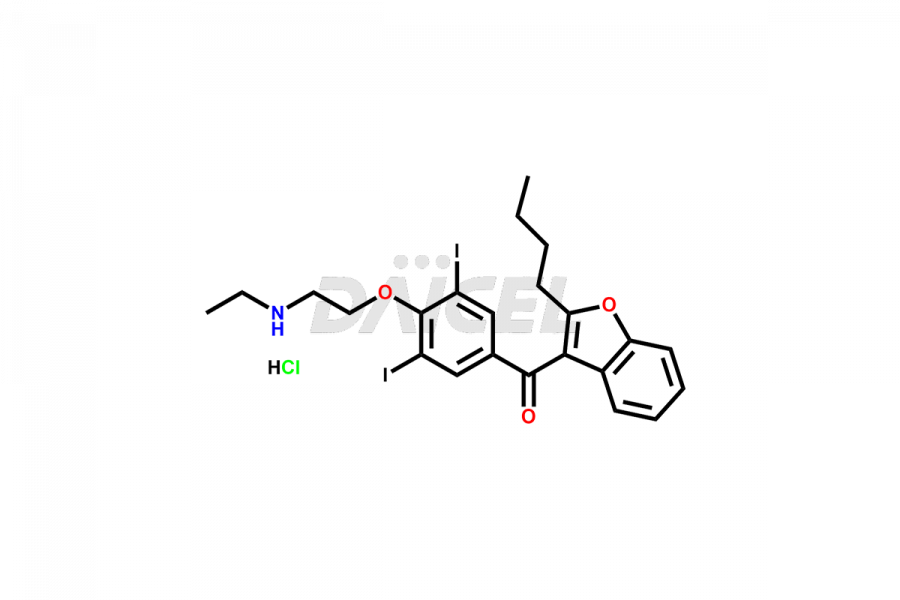
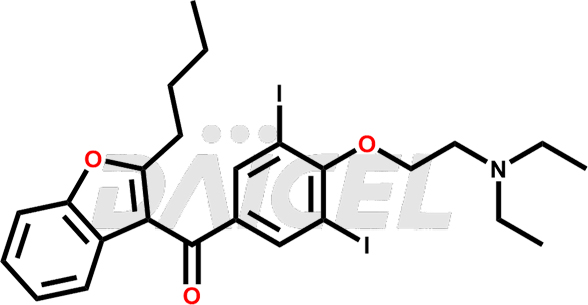
Amiodarone [CAS: 1951-25-3] is a medicine that contains iodine and belongs to the benzofuran derivatives. It has properties that can help regulate heart rhythms and increase blood flow. It is a class III antiarrhythmic agent. Amiodarone treats various heart rhythm disorders.
Amiodarone: Use and Commercial Availability
Amiodarone is an anti-arrhythmic drug used to treat various heart rhythm disorders. It treats life-threatening ventricular arrhythmias. It treats and prevents frequently
recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular
tachycardia (VT) in patients, refractory to other therapy. Amiodarone is available under various brand names, such as Nexterone, Pacerone, etc.
Amiodarone Structure and Mechanism of Action 
The chemical name of Amiodarone is (2-Butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone. Its chemical formula is C25H29I2NO3, and its molecular weight is approximately 645.3 g/mol.
Amiodarone lengthens cardiac action potential, a characteristic of the Vaughan Williams class III effect. It blocks myocardial potassium and sodium channels that prolong refractoriness in the atrioventricular (AV) node.
Amiodarone Impurities and Synthesis
During the synthesis1 of Amiodarone, impurities can form and harm patients. These impurities may lead to the degradation of Amiodarone or byproducts from the synthetic process. It is necessary to control these impurities as they can cause harm, such as liver and lung toxicity, and decrease drug efficacy.
Daicel Pharma provides a Certificate of Analysis (CoA) for Amiodarone impurity standards, including Amiodarone EP Impurity F, Amiodarone Impurity D, and Amiodarone Impurity E. The CoA is from a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN upon request. Daicel Pharma is capable of creating unknown Amiodarone impurities or degradation products. Each delivery comes with a complete characterization report.
References
FAQ's
References
- Huang, Wenbo; Xu, Jing; Liu, Changhui; Chen, Zhiyan; Gu, Yanlong, Lewis Acid-Catalyzed Synthesis of Benzofurans and 4,5,6,7-Tetrahydrobenzofurans from Acrolein Dimer and 1,3-Dicarbonyl Compounds, Journal of Organic Chemistry, Volume: 84, Issue: 5, Pages: 2941-2950, 2019
- Flanagan, R. J.; Storey, G. C. A.; Holt, D. W., Rapid high-performance liquid chromatographic method for the measurement of amiodarone in blood plasma or serum at the concentrations attained during therapy, Journal of Chromatography, Volume: 187, Issue: 2, Pages: 391-8, 1980
Frequently Asked Questions
What methods help detect Amiodarone impurities?
High-performance liquid chromatography (HPLC) and mass spectrometry (MS) help detect and quantify Amiodarone impurities.
Do impurities in Amiodarone drug products cause drug interactions?
Impurities in Amiodarone drug products may cause drug interactions by altering the drug’s pharmacokinetics or interacting with other medicines.
How can Amiodarone impurities be controlled?
Strict manufacturing processes, proper storage conditions, and analytical testing to detect and quantify impurities can control Amiodarone impurities.
Which solvent helps in the analysis of Amiodarone impurities?
Methanol is a solvent used in analyzing many impurities in Amiodarone.
What are the temperature conditions required to store Amiodarone impurities?
Amiodarone impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.