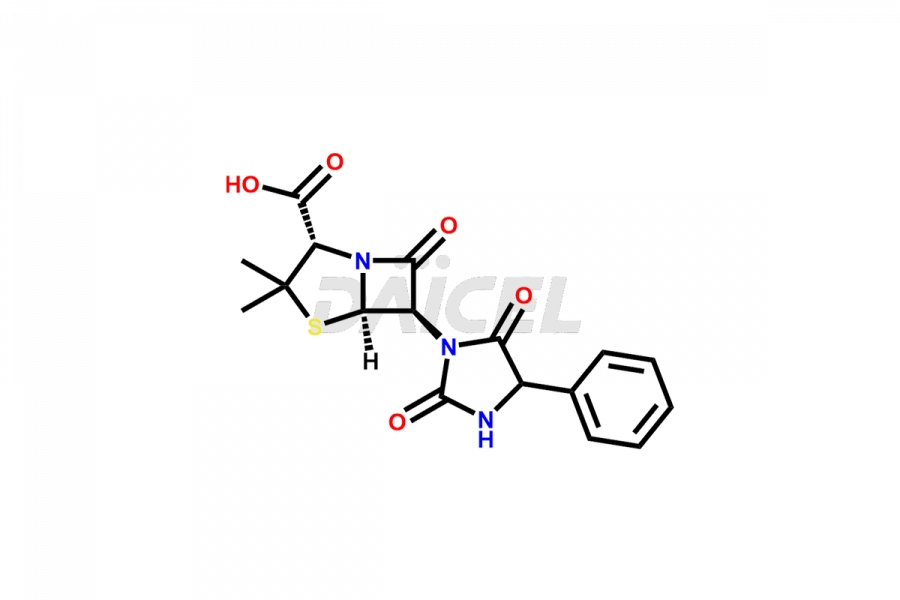
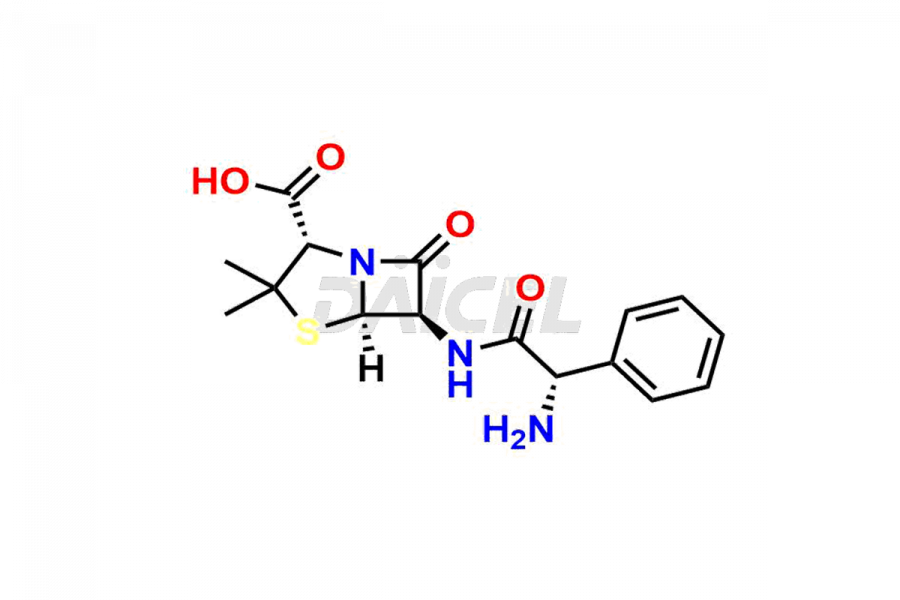
Ampicillin
General Information
Ampicillin Impurities and Ampicillin
Daicel Pharma offers high-quality Ampicillin impurities, Ampicillin hydantoin, and Ampicillin Impurity B. They are vital for the evaluation of the quality, stability, and biological safety of Ampicillin. Furthermore, Daicel Pharma specializes in the custom synthesis of Ampicillin impurities and ensures their worldwide delivery.
Ampicillin [CAS: 69-53-4] is a semi-synthetic derivative of penicillin, a broad-spectrum antibiotic. The penam ring of penicillin is substituted at the 6th position with a 2-amino-2-phenylacetamido group. It is a beta-lactam antibiotic and a known penicillin allergen. Ampicillin is an antibacterial conjugate acid of ampicillin (1-).
Ampicillin: Use and Commercial Availability
Ampicillin, available under various trade names such as Omnipen, Principen, Omnipen-N, and Totacillin-N, is a medication used to treat specific bacterial infections. Ampicillin is synthetically created and classified as a beta-lactam compound. Ampicillin’s stability against degradation by various beta-lactamases enables its utilization in treating many gram-positive and gram-negative infections.
Ampicillin Structure and Mechanism of Action


The chemical name of Ampicillin is (2S,5R,6R)-6-[[(2R)-2-Amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. The chemical formula for Ampicillin is C16H19N3O4S, and its molecular weight is approximately 349.4g/mol.
It exhibits bactericidal effects by binding to penicillin-binding proteins (PBPs) located within the bacterial cell wall’s inner membrane. This binding leads to the inactivation of PBPs, disrupting the cross-linkage of peptidoglycan chains crucial for maintaining bacterial cell wall strength and structure. Consequently, bacterial cell wall synthesis is impeded, weakening the wall and causing cell lysis.
Ampicillin Impurities and Synthesis
Like any pharmaceutical drug, Ampicillin may contain impurities affecting its safety and efficacy. These impurities form during the synthetic process, storage, or drug degradation. Impurities such as L-Ampicillin or Ampicillin Impurity B1 may arise due to traces of L-phenyl glycine methyl ester in D-phenyl glycine methyl ester hydrochloride. Therefore, to ensure the safety and quality of Ampicillin, these impurities must be controlled and monitored throughout the drug’s lifecycle, from production to patient administration.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Ampicillin impurities, which includes Ampicillin hydantoin and Ampicillin Impurity B. This CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We provide additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Ampicillin impurity or degradation product. We can provide labeled compounds to measure the effectiveness of generic Ampicillin. Furthermore, Daicel offers highly purified isotope-labeled standards of Ampicillin. We provide a complete characterization report upon delivery.
References
FAQ's
References
- Chilamkurthi Suresh, Dharmasoth Rama Devi, Balakrishna Aegurla, Aaramadaka Sunil Kumar Reddy, K. V. V. Prasada Rao, Guduri Anil Kumar & Keloth Basavaiah; Synthesis and characterization of potential impurities of Ampicillin trihydrate, Volume 77, pages 7961–7967, (2023)
- Smith, John W. G.; De Grey, G. E.; Patel, V. J.; Spectrophotometric determination of ampicillin, Analyst (Cambridge, United Kingdom), Volume: 92, Issue: 1093, Pages: 247-52, Journal; 1967.DOI: 10.1039/an9679200247
Frequently Asked Questions
What are the common impurities found in Ampicillin?
The common impurities in Ampicillin include Ampicillinyl-D-phenylglycine and L-Ampicillin.
How can the formation of impurities be minimized during the synthetic process?
The formation of impurities can be minimized by optimizing the synthesis process, using high-quality raw materials, and maintaining strict quality control measures.
How are impurities detected in Ampicillin?
Impurities in Ampicillin can be detected using various analytical techniques such as HPLC, NMR, and HRMS.
What is the impact of impurities on the stability of Ampicillin?
Impurities can affect the stability of Ampicillin, leading to a decrease in its shelf life.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.