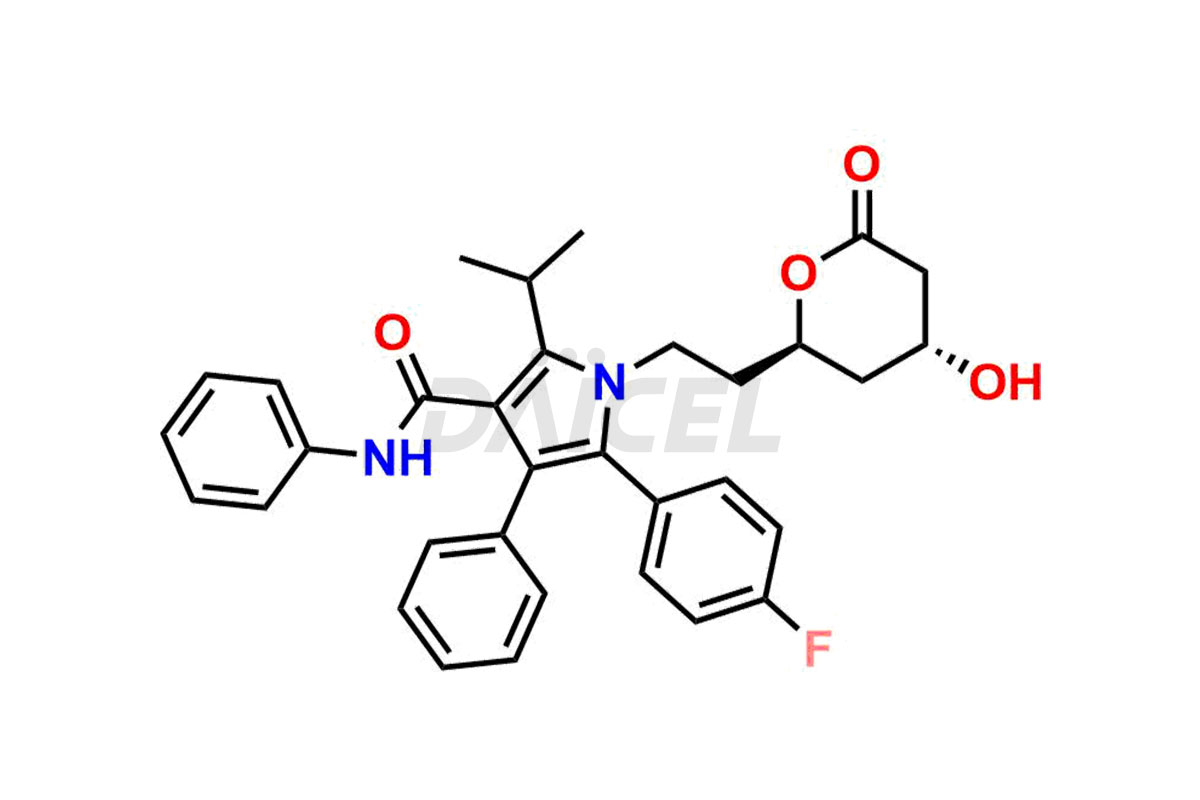
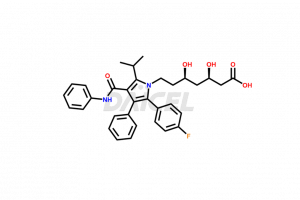
Atorvastatin
General Information
Atorvastatin Impurities and Atorvastatin
Daicel Pharma offers the best-quality Atorvastatin impurities, such as Atorvastatin Lactone. They are vital for evaluating the quality, stability, and biological safety of Atorvastatin. Furthermore, Daicel Pharma specializes in the custom synthesis of Atorvastatin impurities and ensures their worldwide delivery.
Atorvastatin [CAS: 134523-00-5] is a statin that reduces blood cholesterol. It prevents cardiovascular diseases and lowers lipoprotein levels in the liver. It inhibits the rate-limiting enzyme HMG-CoA reductase, which converts 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate. Mevalonate is a precursor of cholesterol. Atorvastatin reduces low-density lipoprotein (LDL) synthesis responsible for cardiovascular diseases.
Atorvastatin: Use and Commercial Availability
As a statin, Atorvastatin is a lipid-lowering medicine. It prevents coronary heart disease. Atorvastatin treats dyslipidemia, wherein the plasma cholesterol and triglycerides rise. Further, It is prescribed with dietary modifications to prevent cardiovascular events in high-risk patients. It acts as a preventive medicine for myocardial infarction, angina, stroke, and revascularization in type-2 diabetic patients with multiple risk factors. Atorvastatin is available under the brands Lipitor and Atorvaliq as oral formulations.
Atorvastatin Structure and Mechanism of Action
The chemical name of Atorvastatin is (3R,5R)-7-[2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid. The chemical formula for Atorvastatin is C33H35FN2O5, and its molecular weight is approximately 558.64 g/mol.
Atorvastatin inhibits 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. It decreases cholesterol synthesis in the liver.
Atorvastatin Impurities and Synthesis
When synthesizing Atorvastatin1, impurities form that may affect the safety and efficacy of the drug. These impurities form during the synthetic process, purification, and storage of Atorvastatin. As a result, Atorvastatin impurities must be controlled and monitored throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Atorvastatin impurities, which includes Atorvastatin Lactone. A cGMP-compliant analytical facility furnishes the CoA. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Atorvastatin impurity or degradation product. In addition, Daicel Pharma offers highly purified stable isotope-labeled standards of Atorvastatin for bioanalytical research and BA/BE studies. Customers can expect a complete characterization report on delivery.
References
- Roth, Bruce David, [R-(R*R*)]-2-(4-fluorophenyl)- beta , delta -dihydroxy-5-(1-methylethyl-3-phenyl-4-[(phenylamino) carbonyl]- 1H-pyrrole-1-heptanoic acid, its lactone form and salts thereof, US5273995A, Feb 26, 1991, Warner-Lambert Co., United States (https://patents.google.com/patent/US5273995A/en)
- Jemal, Mohammed; Ouyang, Zheng; Chen, Bang-Chi; Teitz, Deborah, Quantitation of the acid and lactone forms of atorvastatin and its biotransformation products in human serum by high-performance liquid chromatography with electrospray tandem mass spectrometry, Rapid Communications in Mass Spectrometry, Volume: 13, Issue: 11, Pages: 1003-1015, 1999 DOI: (10.1002/(sici)1097-0231(19990615)13:11<1003::aid-rcm597>3.0.co;2-l)
Frequently Asked Questions
2.How do we characterize the potential known impurities of Atorvastatin?
Analytical methods like 1H NMR, 13C NMR, and MS characterize the potential known impurities of Atorvastatin.
3.Which analytical method identifies Atorvastatin impurities and their quantity in drug substance?
HPLC with a diode array detector identifies Atorvastatin impurities and their quantity in drug substance.
4.Which analytical method detects elemental impurities in Atorvastatin?
The inductively coupled plasma mass spectrometric method detects elemental impurities in Atorvastatin.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.