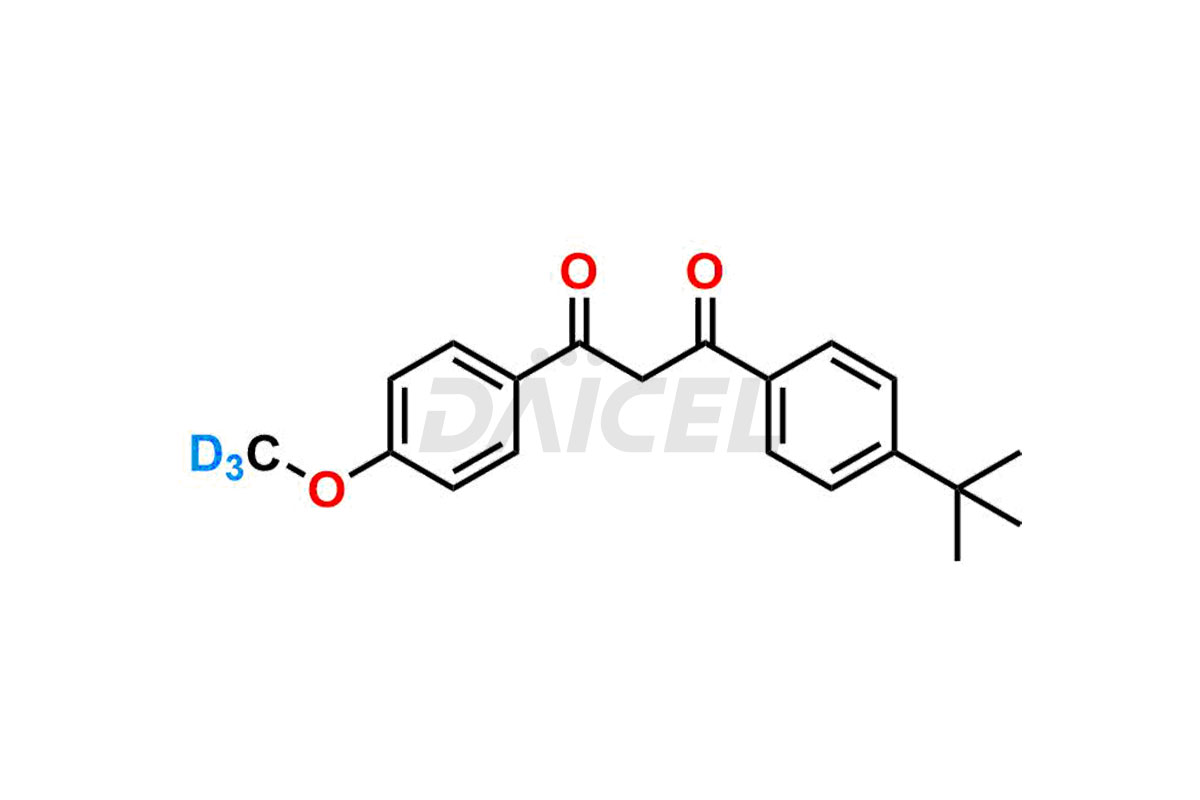
Avobenzone D3
General Information
Avobenzone Impurities and Avobenzone
Daicel Pharma offers superior-quality Avobenzone impurities and Avobenzone D3 Labelled Standard. It is vital for evaluating Avobenzone quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Avobenzone impurities and ensures their worldwide delivery.
Avobenzone [CAS: 70356-09-1] is a sunscreen agent. It blocks Ultraviolet (UVA I, UVA II, and UVB) wavelengths, protecting the skin from harmful UV rays. It is an oil-soluble ingredient used in many sunscreens.
Avobenzone: Use and Commercial Availability
Avobenzone is part of sunscreen formulation. It is a UVA filter absorbing maximum UVA rays. As it is light-sensitive, photo stabilizers are added to the formulation to increase its stability and activity. Further, sunscreen agents reduce symptoms like premature skin aging and skin cancer. It is a broad-range UV protector and is sold as a topical formulation. It is available under brands Parsol 1789, Parsol A, Photoplex, Milestab 1789, etc.
Avobenzone Structure and Mechanism of Action
The chemical name of Avobenzone is 1-[4-(1,1-Dimethylethyl)phenyl]-3-(4-methoxyphenyl)-1,3-propanedione. The chemical formula for Avobenzone is C20H22O3, and its molecular weight is approximately 310.39 g/mol.
Avobenzone absorbs UV radiation within a specific wavelength range and decreases ultraviolet (UV) light penetration through the epidermis.
Avobenzone Impurities and Synthesis
While synthesizing Avobenzone 1, impurities may form that will affect the safety and efficacy of the drug. These impurities form during Avobenzone synthesis, purification, storage, or degradation. Avobenzone impurities need control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Avobenzone impurities and Avobenzone D3 Labelled Standard. A CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Avobenzone impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, such as the Avobenzone D3 Labelled Standard. A complete characterization report is available on delivery.
References
- De Polo, Karl Fred, 4-(1,1-Dimethylethyl)-4'-methoxydibenzoylmethane, US4387089A, May 18, 1981, Givaudan, L., et Cie. S. A., Switzerland (https://patents.google.com/patent/US4387089A/en)
- Ikeda, Kazuo; Suzuki, Sukeji; Watanabe, Yohya, Determination of sunscreen agents in cosmetic products by reversed-phase high-performance liquid chromatography, Journal of Chromatography, Volume: 482, Issue: 1, Pages: 240-5, 1989 DOI: (1016/s0021-9673(01)93225-x)
Frequently Asked Questions
2.What are the photodegraded products of Avobenzone?
2-chloro-1-(4-tert-butylphenyl)-3-(4methoxyphenyl)-1,3-propanedione and 2,2-dichloro-1-(4-tert-butylphenyl)-3-(4methoxyphenyl)-1,3-propanedione are the photodegraded products of Avobenzone.
3.How do we identify photodegraded products of Avobenzone in drug substance?
UV-spectroscopy helps identify photodegraded products of Avobenzone in drug substance.
4.Why is it necessary to remove Avobenzone photodegraded products from the drug substance?
The presence of Avobenzone photodegraded products can impact drug safety and efficacy. Hence, it is crucial to remove Avobenzone photodegraded products from the drug substance.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.