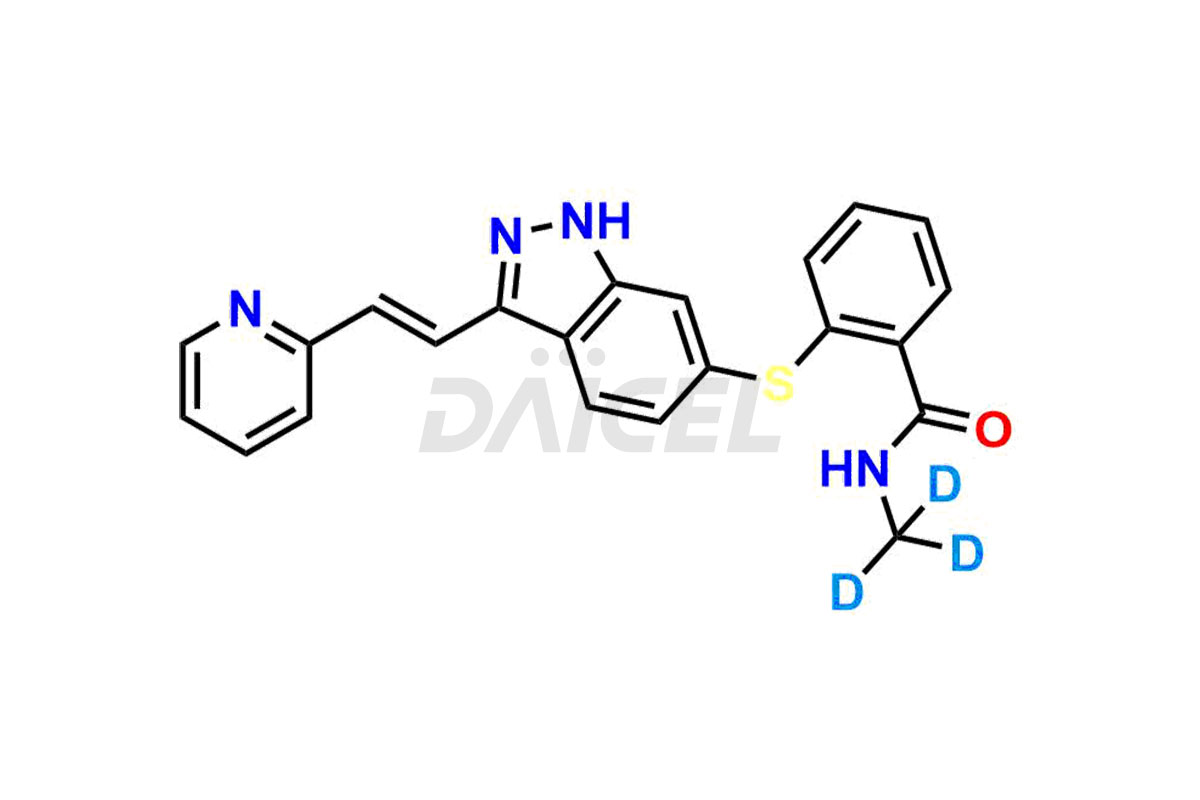
Axitinib
General Information
Axitinib Impurities and Axitinib
Daicel Pharma offers high-quality Axitinib impurities and Axitinib Labelled Standard. It is vital for evaluating Axitinib quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Axitinib impurities and ensures their worldwide delivery.
Axitinib [CAS: 319460-85-0] is an indazole derivative. It treats advanced renal cell carcinoma (RCC). It is indicated after failure of a prior treatment. It is an antineoplastic agent and inhibits tyrosine kinases. It blocks VEGF receptors responsible for tumor growth, cancer progression, and pathologic angiogenesis.
Axitinib: Use and Commercial Availability
Axitinib treats advanced kidney cancer in patients. It treats adult cancer patients with an earlier cancer treatment. It is used alone or with other drugs. Further, it treats different types of cancer. Axitinib blocks the growth of tumors and the progression of cancer. It is available as an oral formulation under the brand Inlyta.
Axitinib Structure and Mechanism of Action
The chemical name of Axitinib is N-Methyl-2-[[3-[(1E)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]benzamide. The chemical formula for Axitinib is C22H18N4OS, and its molecular weight is approximately 386.47 g/mol.
Axitinib blocks receptor tyrosine kinases and vascular endothelial growth factor receptors, such as (VEGFR)-1, VEGFR-2, and VEGFR-3. It inhibits VEGF-mediated endothelial cell proliferation.
Axitinib Impurities and Synthesis
During the synthesis of Axitinib1, impurities form that may affect the safety and efficacy of the drug. These impurities form during the manufacture, storage, or degradation of Axitinib. As a result, Axitinib impurities must be controlled and monitored throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Axitinib impurities and Axitinib Labelled Standard. A cGMP-compliant analytical facility furnishes the CoA. It provides complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare unidentified Axitinib degradation products or impurities. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Axitinib Labelled Standard. Customers can expect a complete characterization report on delivery.
References
- Kania, Robert Steven; Bender, Steven Lee; Borchardt, Allen J.; Braganza, John F.; Cripps, Stephan James; Hua, Ye; Johnson, Michael David; Johnson, Theodore Otto, Jr.; Luu, Hiep The; Palmer, Cynthia Louise; et al, Indazole compounds and pharmaceutical compositions for inhibiting protein kinases, and methods for their use, WO0102369A2, Jan 11, 2001, Agouron Pharmaceuticals, Inc., United States (https://patents.google.com/patent/WO2001002369A2/en)
- Sparidans, Rolf W.; Iusuf, Dilek; Schinkel, Alfred H.; Schellens, Jan H. M.; Beijnen, Jos H., Liquid chromatography-tandem mass spectrometric assay for the light sensitive tyrosine kinase inhibitor axitinib in human plasma, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 877, Issue: 32, Pages: 4090-4096, 2009 DOI: ( 10.1016/j.jchromb.2009.10.024)
Frequently Asked Questions
2.What are the types of degradation products found in Axitinib?
Sulfone, isomeric, sulfoxide, and dimer impurities are found in Axitinib.
3.How do we characterize Axitinib degradation products?
Liquid chromatography-high resolution mass spectrometry and nuclear magnetic resonance spectroscopy are the methods to characterize Axitinib degradation products.
4.What is the source of process-related impurities in Axitinib?
Palladium-catalyzed reagents and residual solvents form process-related impurities in Axitinib.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.