Belumosudil
General Information
Belumosudil Impurities and Belumosudil
Daicel Pharma offers high-quality Belumosudil impurities, Belumosudil Metabolite KD025M2 and Belumosudil-D7. They are vital for evaluating the quality, stability, and biological safety of Belumosudil. Furthermore, Daicel Pharma specializes in the custom synthesis of Belumosudil impurities and ensures their worldwide delivery.
Belumosudil [CAS: 911417-87-3], a promising oral drug, is a selective inhibitor of Rho-associated, coiled-coil-containing protein kinase 2 (ROCK2). It holds the potential for effectively managing chronic graft-versus-host disease (cGVHD), a significant contributor to morbidity and late nonrelapse mortality following allogeneic hematopoietic cell transplantation.
Belumosudil: Use and Commercial Availability
Belumosudil is available orally and acts as an inhibitor of Rho-associated coiled-coil kinase 2 (ROCK2; ROCK-II). It treats adults and pediatric patients 12 and older, with chronic graft-versus-host disease (chronic GVHD). This medication is sold under the brand name Rezurock.
Belumosudil Structure and Mechanism of Action

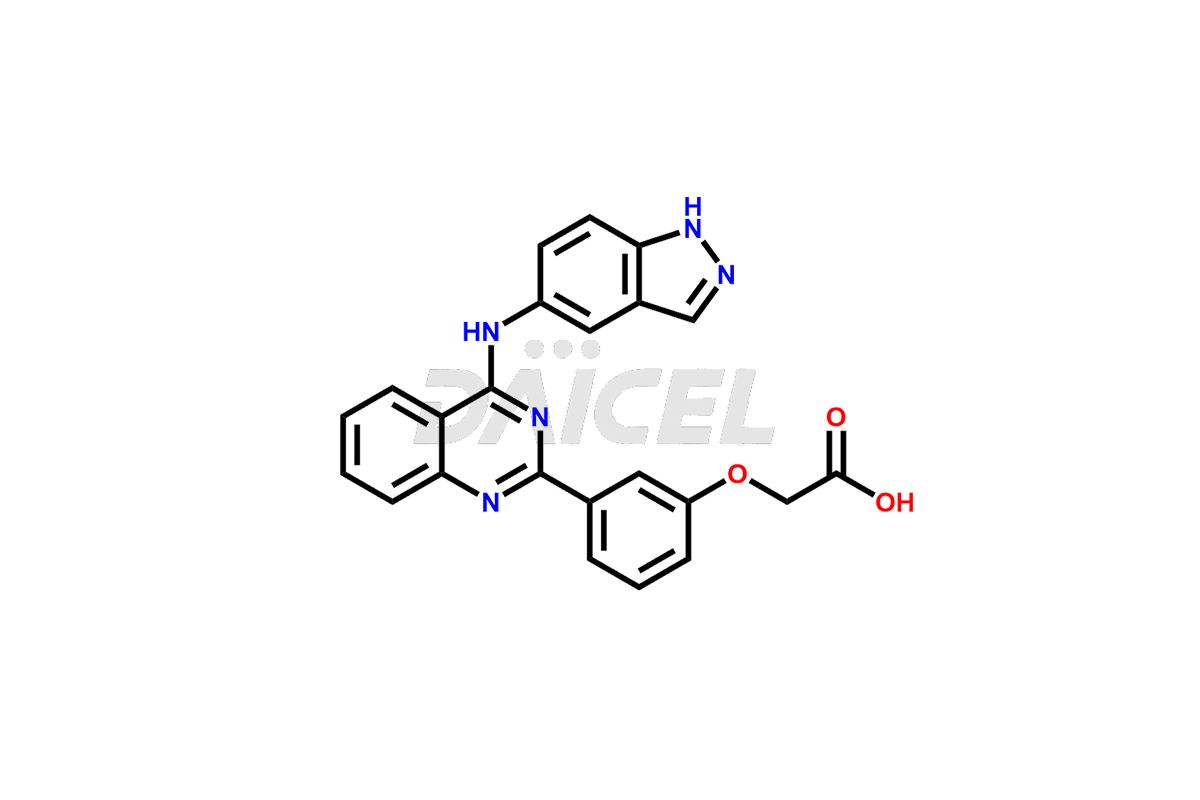
The chemical name of Belumosudil is 2-[3-[4-(1H-Indazol-5-ylamino)-2-quinazolinyl]phenoxy]-N-(1-methylethyl)acetamide. The chemical formula for Belumosudil is C26H24N6O2, and its molecular weight is approximately 452.5g/mol.
Belumosudil inhibits rho-associated, coiled-coil containing protein kinase (ROCK), which stops ROCK2 and ROCK1 with IC50 values of approximately 100 nM and 3µM, respectively. It down-regulates proinflammatory responses via regulation of STAT3/STAT5 phosphorylation. Further, it shifts Th17/Treg balance in ex-vivo or in-vitro-human T cell assays.
Belumosudil Impurities and Synthesis
Belumosudil synthesis1 is necessary to ensure drug purity and efficacy. However, during synthesis, impurities that form potentially compromise safety and effectiveness. Rigorous purification processes, including chromatography and recrystallization, can minimize impurities. Continuous monitoring and stringent quality control measures safeguard the integrity of Belumosudil.
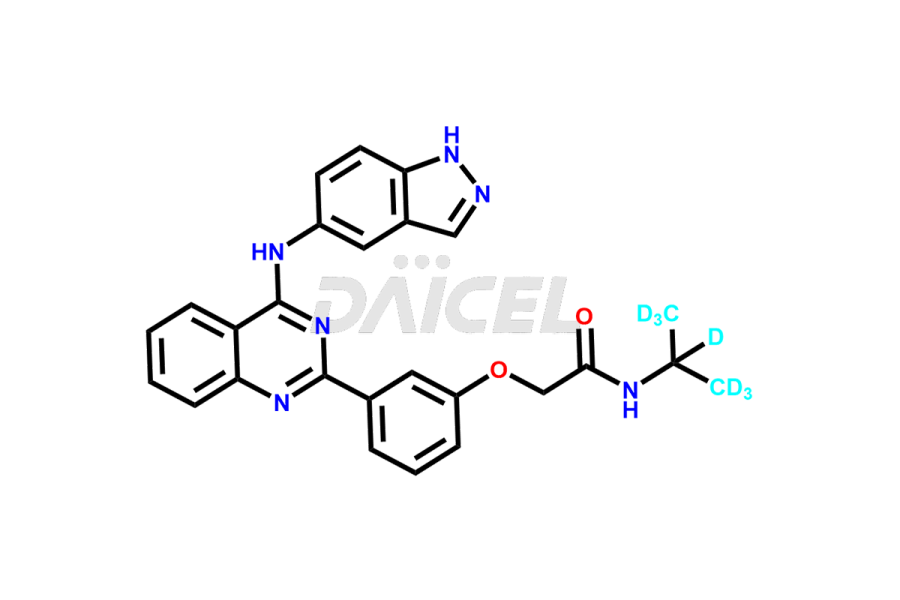
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Belumosudil impurities, such as Belumosudil Metabolite KD025M2. This CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we can give additional data like 13C-DEPT and CHN. Daicel Pharma can also prepare any unidentified Belumosudil impurity or degradation product. Daicel Pharma offers Belumosudil-D7, a deuterium-labeled Belumosudil standard for BA/BE studies. We provide a complete characterization report upon delivery.
References
FAQ's
References
- Campbell, Stewart; Foudoulakis, Hope; Kirk, Brian; Ram, Siya; Bartolozzi, Alessandra; Sweetnam, Paul, Pharmacokinetically Improved Compounds, WO2006105081A2, Oct 5, 2006, Surface Logix, Inc., United States
- Reddy, T. Raja; Namburi, L. A. Amarbabu; Reddy, M. B. Madhusudana, UPLC method determination and quantification of belumosudil and its impurities in the human plasma samples, Rasayan Journal of Chemistry, Volume: 17, Issue: 1, Pages: 207-214, 2024, DOI: (10.31788/rjc.2024.1718697)
Frequently Asked Questions
What are the common types of Belumosudil impurities encountered?
Common Belumosudil impurities include related substances, degradation products, and residual solvents.
Why is it essential to control Belumosudil impurities during synthesis?
Controlling Belumosudil impurities is crucial to ensure the final pharmaceutical product's safety, efficacy, and quality.
What are the storage conditions for Belumosudil to prevent impurity formation?
What are the safety measures when handling Belumosudil impurities in a laboratory?
What are the safety measures when handling Belumosudil impurities in a laboratory?
The safety measures are wearing appropriate personal protective equipment (PPE), working in a well-ventilated area, and following standard operating procedures for handling hazardous chemicals.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.