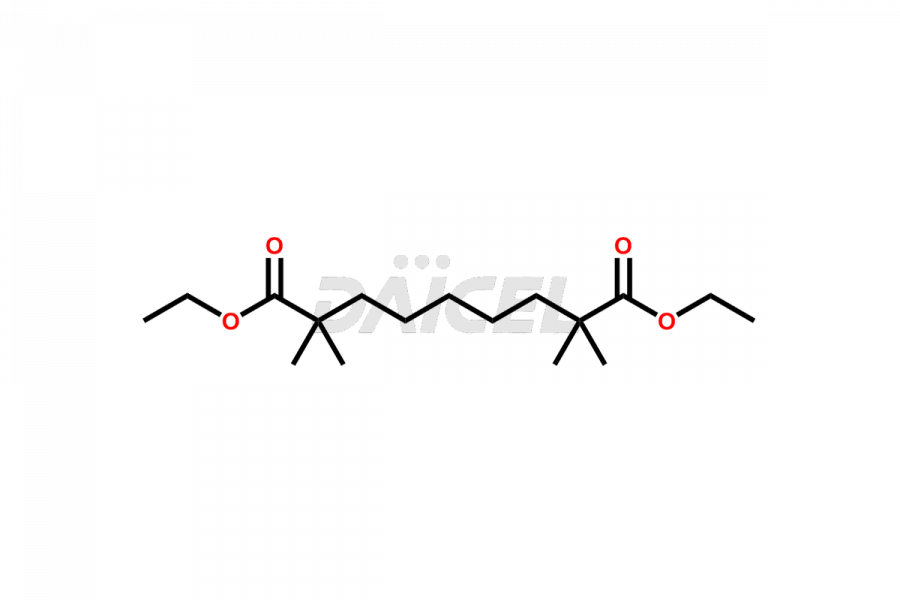
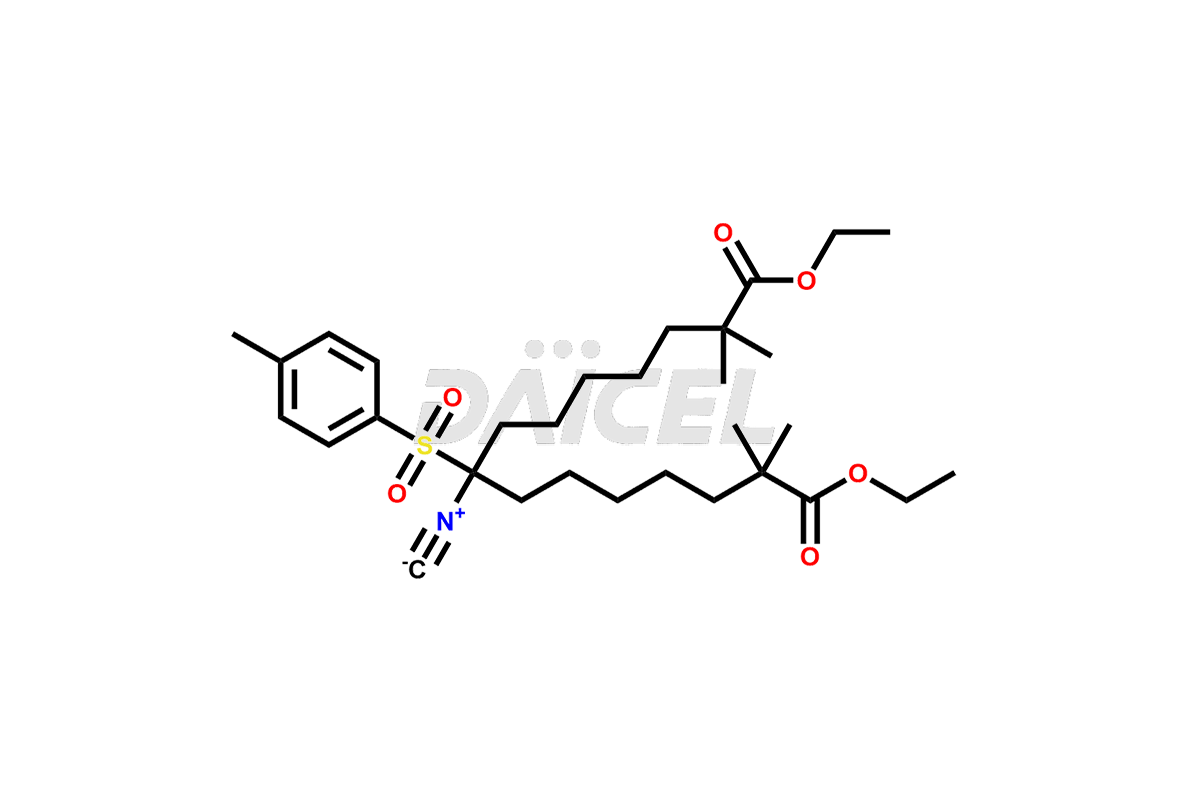
Bempedoic acid
General Information
Bempedoic Acid Impurities and Bempedoic Acid
Daicel Pharma offers high-quality Bempedoic Acid impurities, 2,2,14,14-tetramethyl-8-oxopentadecanedioic acid, 2,2,8,8-tetramethylnonanedioic acid, and Diethyl 8-isocyano-2,2,14,14-tetramethyl-8- tosylpentadecanedioate. They are vital for evaluating the quality, stability, and biological safety of Bempedoic Acid. Furthermore, Daicel Pharma specializes in the custom synthesis of Bempedoic Acid impurities and ensures their worldwide delivery.
Bempedoic acid [CAS: 738606-46-7] is an alpha, omega-dicarboxylic acid known as Pentadecanedioic acid. It has methyl substitutions at positions 2 and 14 and a hydroxy group at position 8. It treats elevated LDL cholesterol levels, often termed ‘bad cholesterol.’ Its functions include acting as an antilipemic drug.
Bempedoic Acid: Use and Commercial Availability
Bempedoic acid is a medication prescribed to decrease the level of low-density lipoprotein cholesterol (LDL-C) in the bloodstream of patients. It has received approval from the U.S. Food and Drug Administration (FDA). Moreover, Bempedoic acid offers an alternative to patients’ intolerant to statins. This medication is marketed under the brand name Nexletol.
Bempedoic acid Structure and Mechanism of Action

The chemical name of Bempedoic acid is 8-Hydroxy-2,2,14,14-tetramethylpentadecanedioic acid. The chemical formula for Bempedoic acid is C19H36O5, and its molecular weight is approximately 344.5g/mol.
Bempedoic acid is an adenosine triphosphate-citrate lyase (ACL) inhibitor. It reduces low-density lipoprotein cholesterol (LDL-C) by inhibiting cholesterol synthesis in the liver.
Bempedoic acid Impurities and Synthesis
Bempedoic acid synthesis1 involves complex chemical processes that can yield impurities. These impurities, if not carefully managed, may compromise the drug’s quality and safety. Purification techniques such as chromatography and crystallization minimize impurities and ensure the integrity of Bempedoic Acid. Continuous monitoring and adherence to strict quality control protocols are essential during synthesis for efficacy.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Bempedoic acid impurities, which includes 2,2,14,14-tetramethyl-8-oxopentadecanedioic acid, 2,2,8,8-tetramethylnonanedioic acid, and Diethyl 8-isocyano-2,2,14,14-tetramethyl-8- tosylpentadecanedioate. The CoA is from a cGMP-compliant analytical facility and, encompasses complete characterization data such as 1H NMR, 13C NMR2, IR, MASS, and HPLC purity. On request, we give additional data like 13C-DEPT and CHN can be provided. Daicel Pharma prepares any unidentified Bempedoic acid impurity or degradation product. Furthermore, Daicel Pharma offers highly purified isotope-labeled standards of Bempedoic acid for BA/BE studies. We also provide a complete characterization report upon delivery.
References
FAQ's
References
- Dasseux, Jean-Louis Henri; Oniciu, Daniela Carmen, Preparation of hydroxyl compounds for cholesterol management and related uses, WO2004067489, Aug 12, 2004, Esperion Therapeutics, Inc., United States
- Engel, Brian J.; Preusch, Karen; Brown, Cameron; Cramer, Clay T.; Shoup, Ronald, Measurement of bempedoic acid and its keto metabolite in human plasma and urine using solid phase extraction and electrospray LC-MS/MS, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 1154, Pages: 122291, 2020, DOI: (10.1016/j.jchromb.2020.122291)
Frequently Asked Questions
What are the potential risks associated with the presence of Bempedoic acid impurities?
The risks associated with the presence of Bempedoic Acid impurities include toxicity, reduced therapeutic efficacy, and adverse effects on patient health.
How can Bempedoic acid impurities be minimized during synthesis?
Impurities can be minimized by optimizing the synthetic process, using high-quality starting materials, implementing effective purification techniques, and conducting thorough quality control tests.
Are there specific methods available for removing Bempedoic acid impurities?
Yes, purification techniques such as recrystallization, chromatography, and filtration can remove Bempedoic Acid impurities.
How frequently should impurity levels in Bempedoic Acid be monitored during synthesis?
Impurity levels in Bempedoic Acid should be monitored regularly throughout the synthetic process to ensure compliance with regulatory standards and maintain product quality.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.