LOAD MORE
You're viewed 9 of 11 products
Daicel Pharma synthesizes high-quality Betamethasone impurities, including Betamethasone 17 propionate 21 mesylate Imp-I, Betamethasone DB XI, Betamethasone Dipropionate, Betamethasone Monopropionate, Betamethasone Tripropionate, Betamethasone-DB11 Dipropionate, and Betamethasone-DB11 Monopropionate. These impurities are essential for evaluating the quality, stability, and safety of an active pharmaceutical ingredient, Betamethasone. Additionally, Daicel Pharma offers a customized synthesis of Betamethasone impurities for global delivery to meet the specific needs of our customers.
Betamethasone [CAS: 378-44-9] is a synthetic glucocorticoid having anti-inflammatory, immunosuppressive, and metabolic activities. It stimulates the production of anti-inflammatory proteins and inhibits the synthesis of inflammatory mediators, thus, reducing chronic inflammation and autoimmune reactions.
Betamethasone is a corticosteroid and treats many inflammatory conditions. As a topical medication, it relieves symptoms of skin disorders that respond to corticosteroids. When combined with vitamin D analogs such as calcipotriene, Betamethasone treats plaque psoriasis. In addition to topical use, Betamethasone is also available as an injectable suspension to manage inflammatory conditions, including endocrine, gastrointestinal, and rheumatic disorders. The drug is available under the trade name Celestone. Further, it occurs as various salts or in combination with other medicines under brands like Alphatrex, Beta-Val, Dermabet, Taclonex, etc.
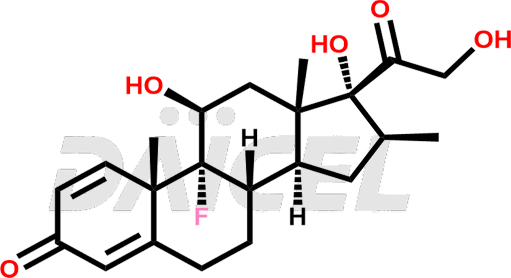
The chemical name of Betamethasone is (11β,16β)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione. Its chemical formula is C22H29FO5, and its molecular weight is approximately 392.5 g/mol.
Corticosteroids contain steroid hormones released by the adrenal cortex and have anti-inflammatory and immunosuppressive properties. The exact mechanism of Betamethasone in different diseases is uncertain.
During the manufacturing1 of Betamethasone, impurities arise, including related compounds, residual solvents, and process impurities, which can impact the drug’s safety and efficacy, stability, and shelf-life of the final product. So, it is essential to control the formation of impurities during the synthesis of Betamethasone to ensure drug quality and purity. Further, appropriate manufacturing procedures, purification techniques, and analytical methods help detect and quantify impurities.
Daicel Pharma provides a Certificate of Analysis (CoA) for Betamethasone impurity standards, including Betamethasone 17 propionate 21 mesylate Imp-I, Betamethasone DB XI, Betamethasone Dipropionate, Betamethasone Monopropionate, Betamethasone Tripropionate, Betamethasone-DB11 Dipropionate, and Betamethasone-DB11 Monopropionate. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of synthesizing unknown Betamethasone impurities or degradation products. Each delivery has a complete characterization report.
The analytical methods that detect Betamethasone impurities include chromatography, spectroscopy, and mass spectrometry. These methods help identify and quantify impurities in the drug substance or drug product.
It is essential to control Betamethasone impurities to ensure the safety and efficacy of the drug product. They can affect the quality and purity of the drug, as well as its stability and shelf-life.
Methanol is the solvent used in analyzing many impurities in Betamethasone.
Betamethasone impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.