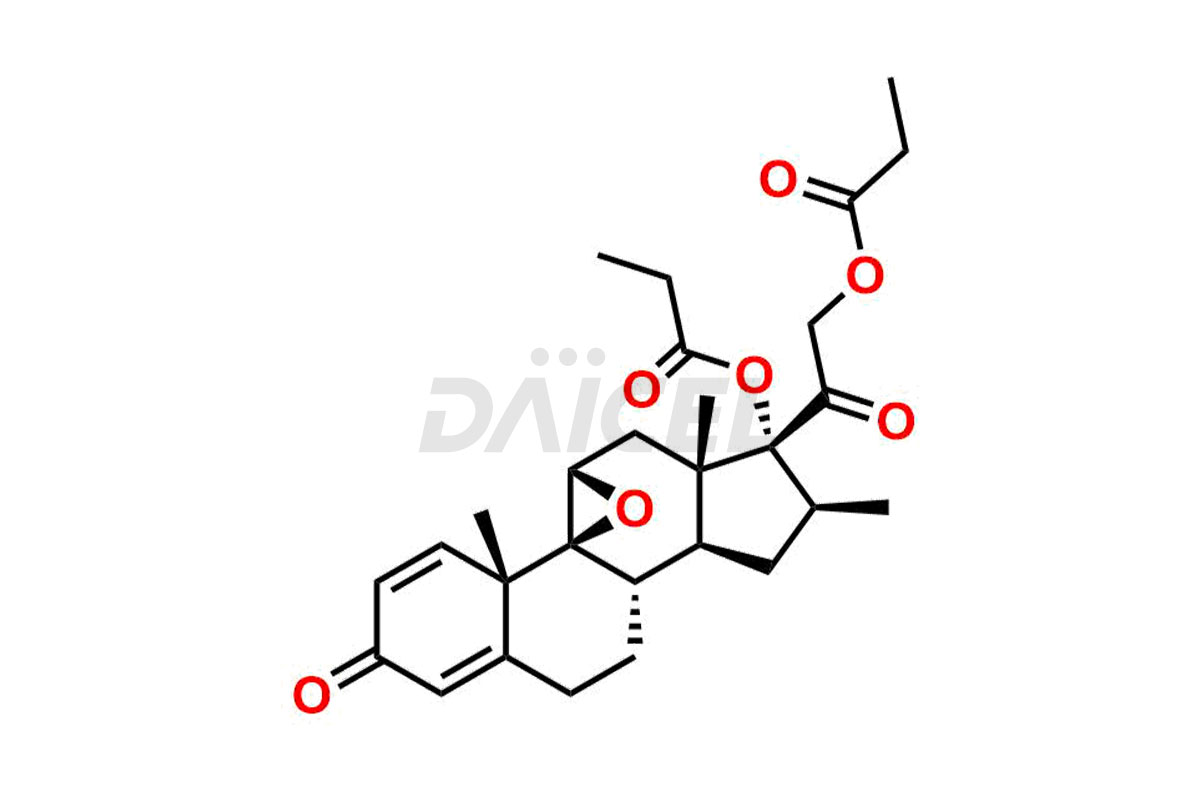
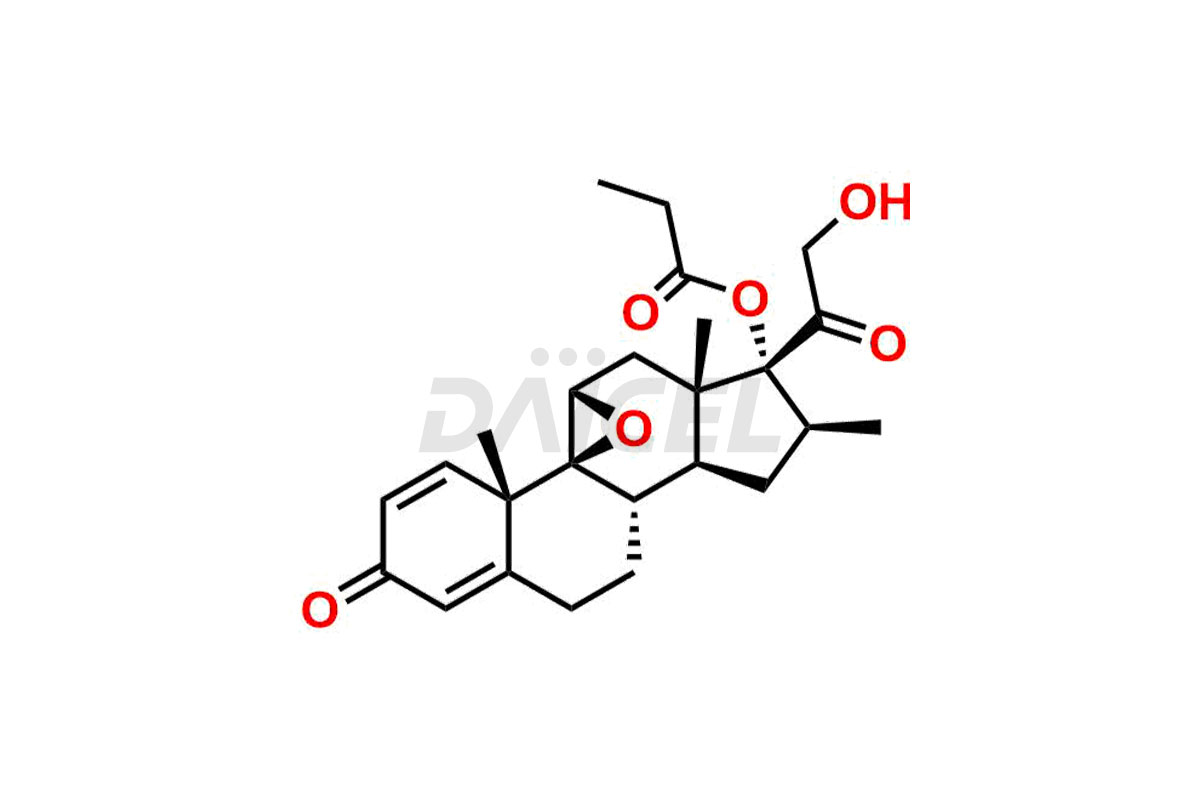
Betamethasone
References
- Taub, D.; Hoffsommer, R. D.; Slates, H. L.; Kuo, C. H.; Wendler, N. L., 16β-Methyl cortical steroids, Journal of the American Chemical Society, Volume: 82, Pages: 4012-26, 1960
- Graham, R. E.; Biehl, E. R.; Kenner, C. T, Analysis of betamethasone and its organic esters in pharmaceutical products, Journal of Pharmaceutical Sciences, Volume: 67, Issue: 3, Pages: 360-3, 1978
Frequently Asked Questions
What are the analytical methods used to detect Betamethasone impurities?
The analytical methods that detect Betamethasone impurities include chromatography, spectroscopy, and mass spectrometry. These methods help identify and quantify impurities in the drug substance or drug product.
Why is it essential to control Betamethasone impurities?
It is essential to control Betamethasone impurities to ensure the safety and efficacy of the drug product. They can affect the quality and purity of the drug, as well as its stability and shelf-life.
Which solvent helps in the analysis of Betamethasone impurities?
Methanol is the solvent used in analyzing many impurities in Betamethasone.
What are the temperature conditions required to store Betamethasone impurities?
Betamethasone impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.