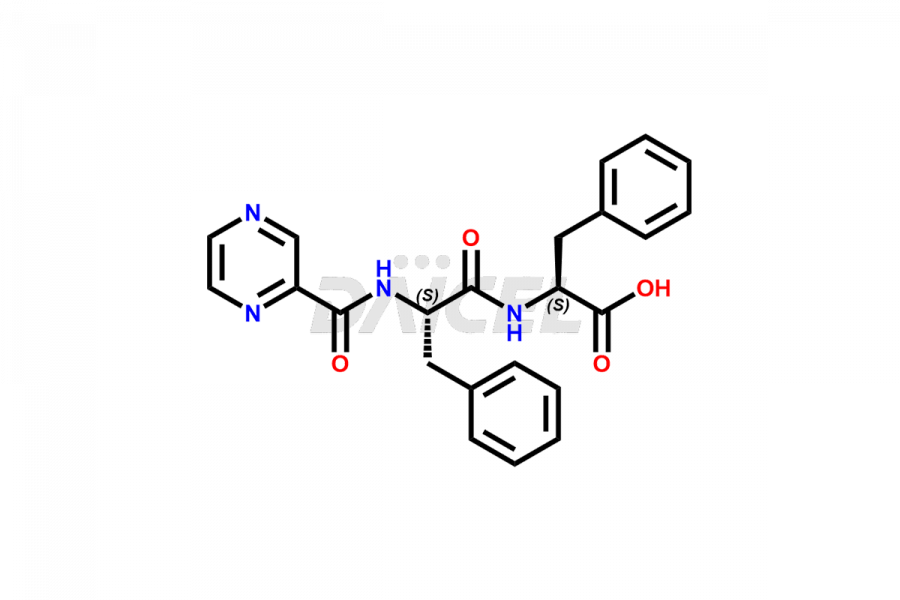
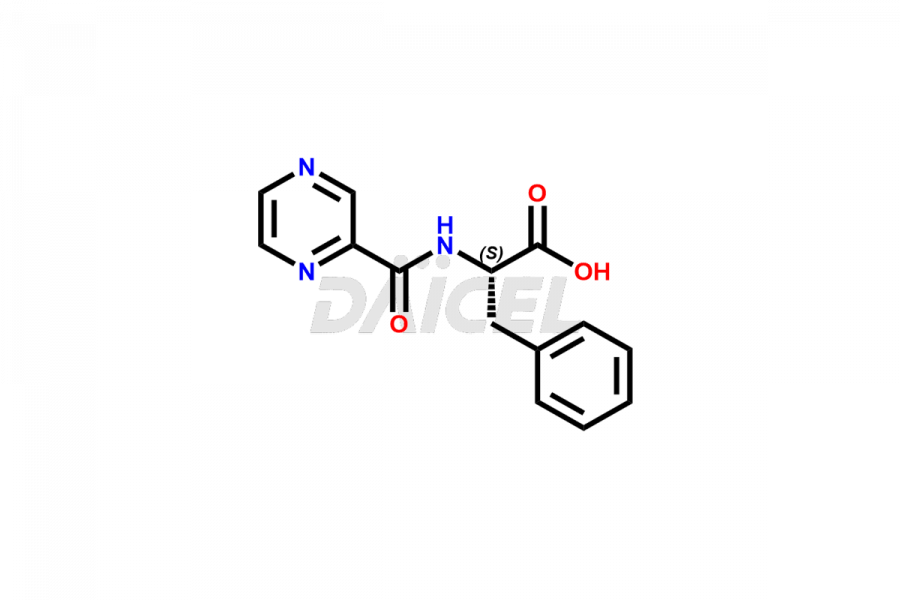
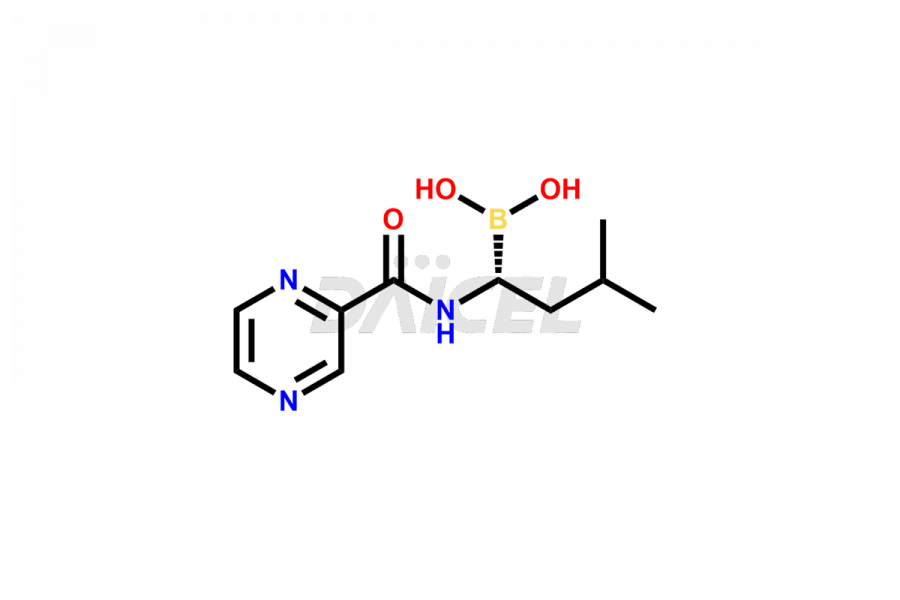
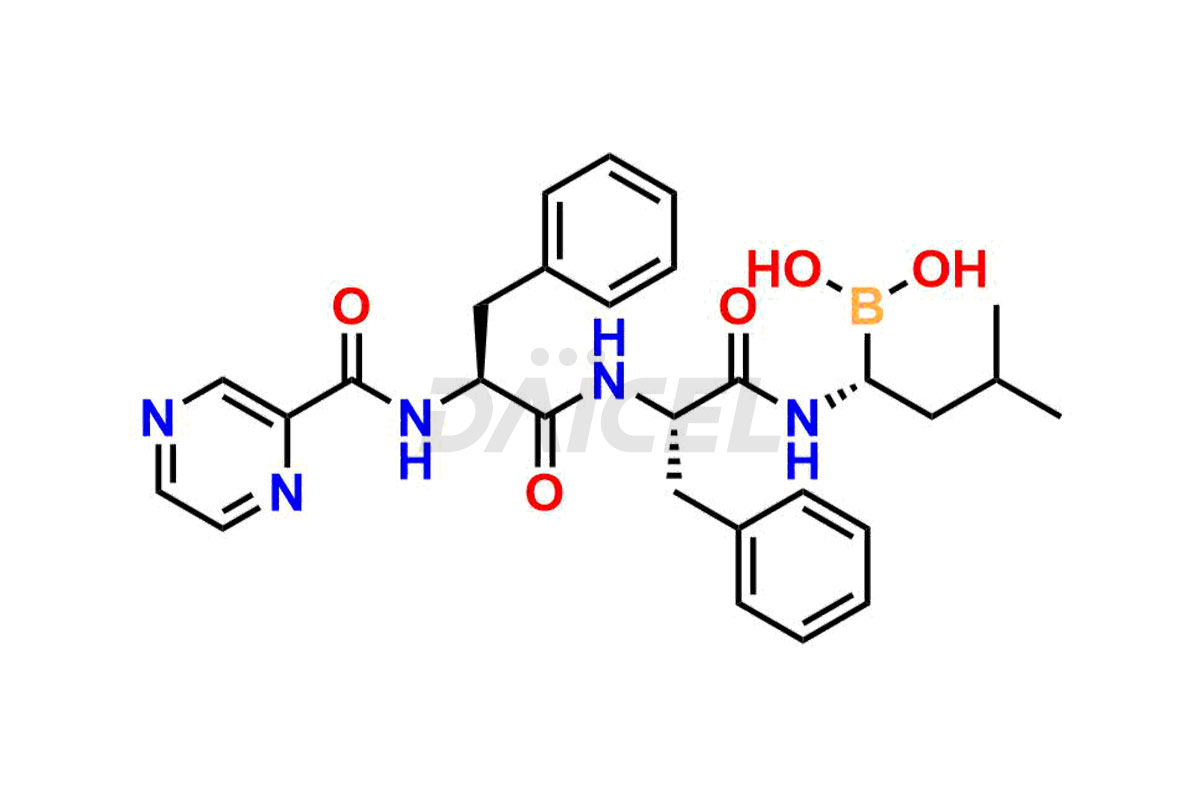
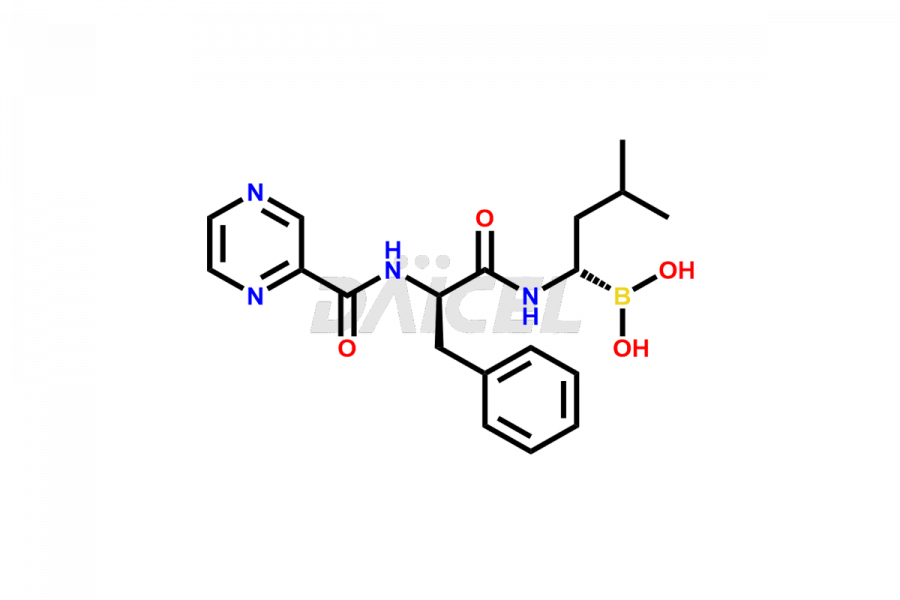
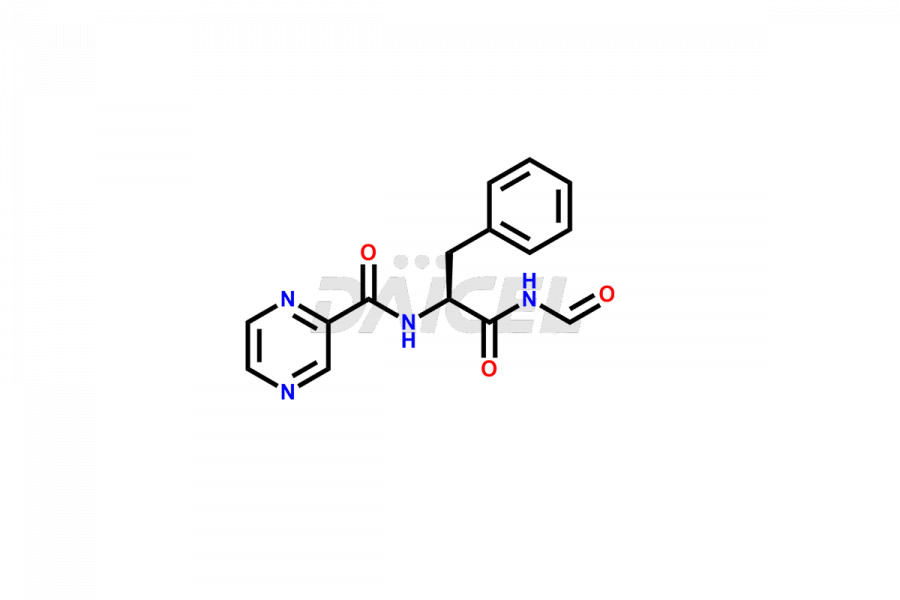
Bortezomib
General Information
Bortezomib Impurities and Bortezomib
Daicel Pharma synthesizes high-quality Bortezomib impurities, including Bortezomib acid impurity, Bortezomib amide impurity, Bortezomib Dimer, and Bortezomib enantiomer. These impurities are essential for evaluating the quality, stability, and safety of an active pharmaceutical ingredient, Bortezomib. Additionally, Daicel Pharma offers a customized synthesis of Bortezomib impurities for delivery globally to meet the specific needs of our customers.
Bortezomib [CAS: 179324-69-7] is an antineoplastic agent and proteasome inhibitor that treats lymphomas and refractory multiple myeloma. It is a modified dipeptidyl boronic acid derivative treating multiple myeloma.
Bortezomib: Use and Commercial Availability
Bortezomib is an FDA-approved antineoplastic agent treating multiple myeloma. It also treats mantle cell lymphoma in patients, undergone at least one prior first-line treatment. Velcade is the brand name under which Bortezomib is available.
Bortezomib Structure and Mechanism of Action
The chemical name of Bortezomib is [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]- Boronic acid. Its chemical formula is C19H25BN4O4, and its molecular weight is approximately 384.2 g/mol.
Bortezomib inhibits the 26S proteasome in mammalian cells, which prevents proteolysis. It disrupts homeostatic mechanisms leading to cell death and delays tumor growth.
Bortezomib Impurities and Synthesis
The impurities that form during the manufacturing1 process or storage of Bortezomib include degradation products and process-related impurities, solvent residues, and residual metals. They can affect the drug safety, efficacy, and quality. So, it is necessary to control them by implementing adequate measures such as using high-quality starting materials, optimizing the manufacturing process, employing appropriate storage conditions, and conducting rigorous quality control tests to ensure the purity and safety of the drug product.
Daicel Pharma provides a Certificate of Analysis (CoA) for Bortezomib impurity standards, including Bortezomib acid impurity, Bortezomib amide impurity, Bortezomib Dimer, and Bortezomib enantiomer. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of creating unknown Bortezomib impurities or degradation products. Each delivery has a complete characterization report.
References
FAQ's
References
- Adams, Julian; Ma, Yu-Ting; Stein, Ross; Baevsky, Matthew; Grenier, Louis; Plamondon, Louis, Boronic ester and acid compounds, synthesis and uses, Proscript, Inc., United States, US6083903A, July 4, 2000
- Byrn, Stephen R.; Tishmack, Patrick A.; Milton, Mark J.; Velde, Helgi, Analysis of Two Commercially Available Bortezomib Products: Differences in Assay of Active Agent and Impurity Profile, AAPS PharmSciTech, Volume: 12, Issue: 2, Pages: 461-467, 2011
Frequently Asked Questions
Can Bortezomib impurities affect the safety and efficacy of the drug product?
Yes, Bortezomib impurities can affect the safety and efficacy of the drug product and may harm patients.
What is the role of quality control in ensuring control of Bortezomib impurities?
Quality control plays a critical role in ensuring that Bortezomib impurities are controlled by testing and analyzing the drug substance and product to identify and quantify any impurities that may be present.
Which solvent helps in the analysis of Bortezomib impurities?
Methanol is a solvent used in analyzing many impurities in Bortezomib.
What are the temperature conditions required to store Bortezomib impurities?
Bortezomib impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.