LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma synthesizes Bosutinib impurities of exceptional quality, such as Bosutinib Impurity-I, Bosutinib Impurity-II, Bosutinib Impurity-III, Bosutinib Impurity-IV, Bosutinib Impurity-V, Bosutinib Impurity-VI, and Oxy Dechlorinated Bosutinib. These impurities are crucial to assess the purity, reliability, and safety of Bosutinib, an active pharmaceutical ingredient. Besides, Daicel Pharma provides a custom synthesis of Bosutinib impurities to meet clients’ demands for delivery worldwide.
Bosutinib [CAS: 380843-75-4] is a medication to treat Philadelphia chromosome-positive chronic myelogenous leukemia in patients. It is a synthetic quinolone derivative and a dual kinase inhibitor of BCR-ABL and Src tyrosine kinases. Bosutinib can potentially prevent cancer cells from growing and spreading by blocking enzymes.
Bosutinib, under the brand Bosulif, is a medication approved for treating adult patients diagnosed with chronic, accelerated, or blast-phase Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML). It helps to control the growth and spread of the leukemia cells in CML.
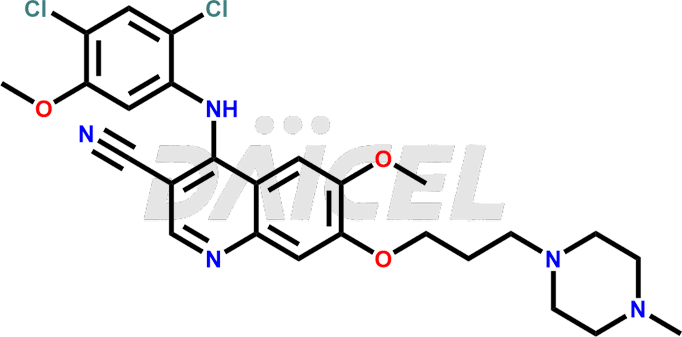
The chemical name of Bosutinib is 4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3-quinolinecarbonitrile. Its chemical formula is C26H29Cl2N5O3, and its molecular weight is approximately 530.4 g/mol.
Bosutinib inhibits Bcr-Abl kinase and Src-family kinases. It inhibits 16 of 18 imatinib-resistant forms of Bcr-Abl expressed in murine myeloid cell lines.
Impurities in Bosutinib are organic or inorganic, process or drug-related. Organic impurities can form during synthesis1, degradation, or storage of the drug substance, while inorganic impurities generate during manufacturing. They can affect the drug’s quality and stability, so their levels must be controlled and minimized during synthesis.
Daicel Pharma offers a Certificate of Analysis (CoA) for Bosutinib impurity standards, such as Bosutinib Impurity-I, Bosutinib Impurity-II, Bosutinib Impurity-III, Bosutinib Impurity-IV, Bosutinib Impurity-V, Bosutinib Impurity-VI, and Oxy Dechlorinated Bosutinib, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can prepare unknown Bosutinib impurities or degradation products. A complete characterization report accompanies every delivery.
Controlling impurities in Bosutinib is essential because they can affect drug safety and efficacy. They can also cause adverse reactions and impact the quality of the drug product.
Bosutinib impurities are controlled during manufacturing using validated analytical methods, monitoring reaction conditions, and implementing quality control measures.
It may not be possible to remove all Bosutinib impurities. However, their control is within acceptable limits through rigorous manufacturing and quality control processes.
Bosutinib impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.