LOAD MORE
You're viewed 9 of 13 products
Daicel Pharma synthesizes Cabotegravir impurities of exceptional quality, such as Cabotegravir impurity 1, Cabotegravir Intermediate-1, Cabotegravir RR Isomer, Cabotegravir RS Isomer, and Cabotegravir SS Isomer. These impurities are crucial to assess the purity, reliability, and safety of Cabotegravir, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Cabotegravir impurities to meet clients’ demands for worldwide delivery.
Cabotegravir [CAS: 1051375-10-0], also known as GSK1265744A, is a medication for treating HIV-1 infection. It is an integrase inhibitor, often prescribed alongside the non-nucleoside reverse transcriptase inhibitor rilpivirine. Cabotegravir helps in the treatment of HIV-1 infection in individuals who have viral suppression.
Cabotegravir is a prescription drug in two forms and is available under two brand names. Vocabria is an oral tablet in combination with another HIV medication called rilpivirine (Edurant) for short-term HIV-1 treatment in adults, virologically suppressed. Apretude is an extended-release injectable form of Cabotegravir that treats at-risk adults and adolescents weighing at least 35 Kg for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 infection.
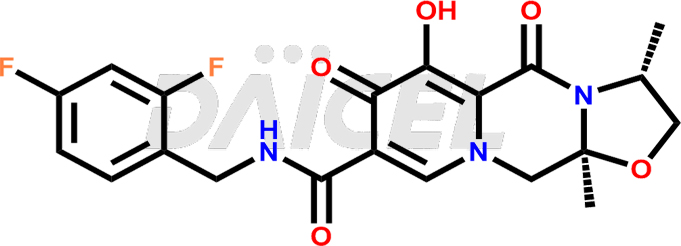
The chemical name of Cabotegravir is (3S,11aR)-N-[(2,4-Difluorophenyl)methyl]-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxooxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide. Its chemical formula is C19H17F2N3O5, and its molecular weight is approximately 405.4 g/mol.
Cabotegravir binds to the HIV integrase active site and inhibits HIV integrase. It blocks the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration for the HIV replication cycle.
Like any other pharmaceutical substance, Cabotegravir may contain impurities that affect its safety and efficacy. These impurities form during the synthesis1, purification, and storage of the drug substance. They can include process-related impurities, such as starting materials, reagents, intermediates, and degradation products that arise due to the chemical or physical instability of the drug substance or its formulation. The presence of impurities can pose potential risks to patients, such as toxicity, reduced potency, or adverse effects. So, it is essential to control and monitor the levels of impurities in Cabotegravir to ensure that it meets the quality and safety standards required for pharmaceutical products.
Daicel Pharma offers a Certificate of Analysis (CoA) for Cabotegravir impurity standards, such as Cabotegravir impurity 1, Cabotegravir Intermediate-1, Cabotegravir RR Isomer, Cabotegravir RS Isomer, and Cabotegravir SS Isomer, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Cabotegravir impurities or degradation products. A complete characterization report accompanies every delivery.
Impurities in Cabotegravir are detected and analyzed using analytical techniques such as high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS). These techniques help in the identification and quantification of impurities.
Cabotegravir impurities can affect the stability of the drug product with an impact on the potency and efficacy of the drug. Controlling impurities is essential to ensure the stability and shelf-life of the drug product.
Acetonitrile or Methanol are the solvents used for analyzing many impurities in Cabotegravir.
Cabotegravir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.