LOAD MORE
You're viewed 9 of 18 products
Daicel Pharma synthesizes Cabozantinib impurities of exceptional quality, such as Cabozantinib monohydroxy sulfate, Cabozantinib N-oxide, Cabozantinib amide cleavage product, CBT Dimer, CBT 2-fluoro, CBT 3-fluoro, and so on. These impurities are crucial to assess the purity, reliability, and safety of Cabozantinib, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Cabozantinib impurities to meet clients’ demands for delivery worldwide.
Cabozantinib [CAS: 849217-68-1] is a small molecule receptor tyrosine kinase (RTK) inhibitor that can fight cancer. This potent antineoplastic agent treats advanced forms of medullary thyroid cancer and renal cell carcinoma resistant to other treatments. Cabozantinib is a promising therapeutic option for patients with metastatic cancer.
Cabozantinib treats advanced cancer like metastatic medullary thyroid cancer and advanced renal cell carcinoma in patients previously undergone antiangiogenic therapy. Additionally, it is approved for use in patients with advanced hepatocellular carcinoma treated with sorafenib. Cabozantinib is available under two brand names: Cabometyx and Cometriq.
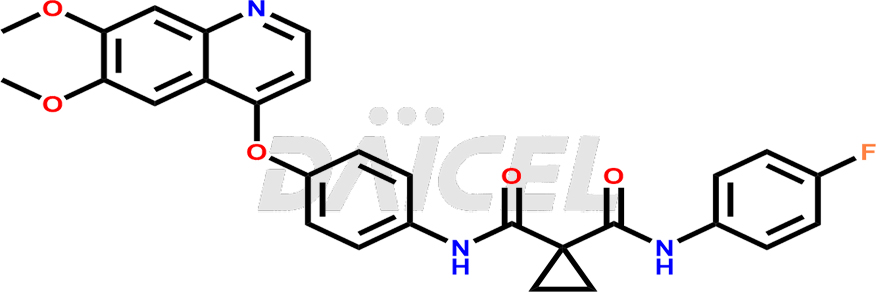
The chemical name of Cabozantinib is N′-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N-(4-fluorophenyl)-1,1-cyclopropane dicarboxamide. Its chemical formula is C28H24FN3O5, and its molecular weight is approximately 501.5 g/mol.
Cabozantinib inhibits the tyrosine kinase activity of receptor tyrosine kinases. They inhibit tumor growth and lead to tumor regression.
During the manufacturing1 of Cabozantinib, impurities form that can be unwanted or potentially harmful. It needs control to ensure the safety and efficacy of the final product. Cabozantinib impurities form through several reactions, like oxidation, hydrolysis, and photolysis. Methods such as process optimization, purification, and analytical testing help control and minimize the formation of impurities in Cabozantinib.
Daicel Pharma offers a Certificate of Analysis (CoA) for Cabozantinib impurity standards, such as Cabozantinib monohydroxy sulfate, Cabozantinib N-oxide, Cabozantinib amide cleavage product, CBT Dimer, CBT 2-fluoro, CBT 3-fluoro, and so on, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Cabozantinib impurities or degradation products2. A complete characterization report accompanies every delivery.
Cabozantinib impurities can have unknown pharmacological effects and can potentially harm patients, so it's essential to identify and control them.
Cabozantinib impurities cannot be removed from the drug product, but their levels can be minimized through effective manufacturing processes and monitoring.
Cabozantinib impurities are monitored throughout the manufacturing process and over the shelf life of the drug product to ensure that their levels remain within acceptable limits.
Cabozantinib impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.