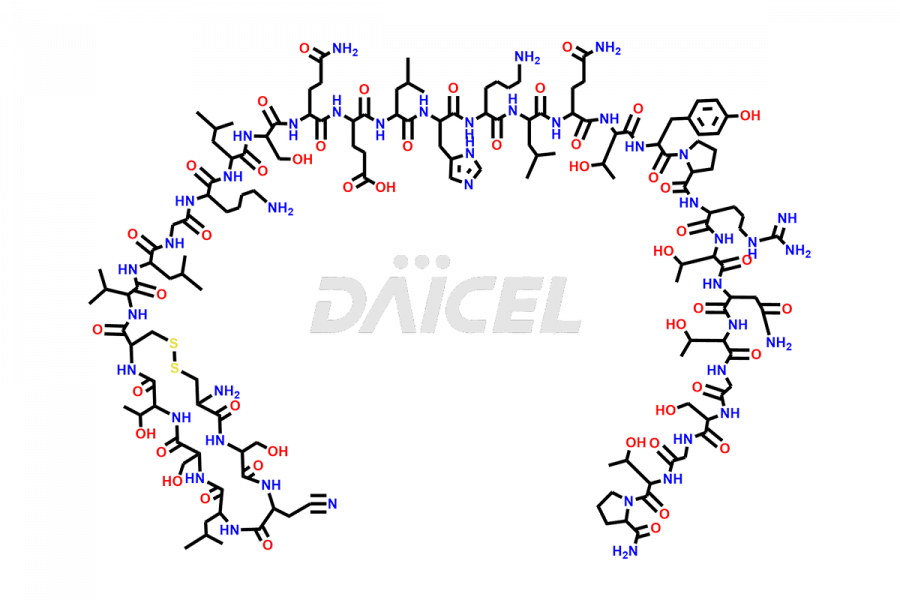
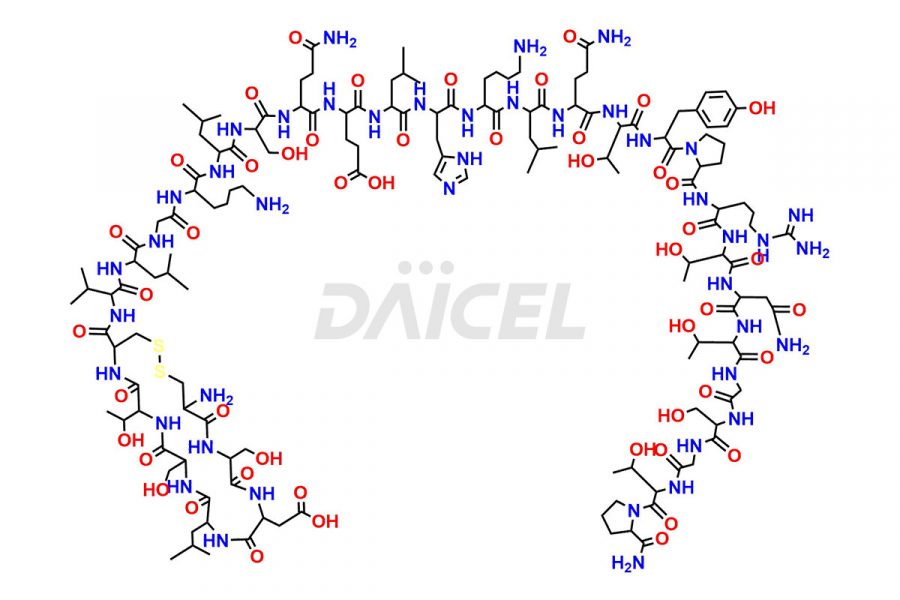
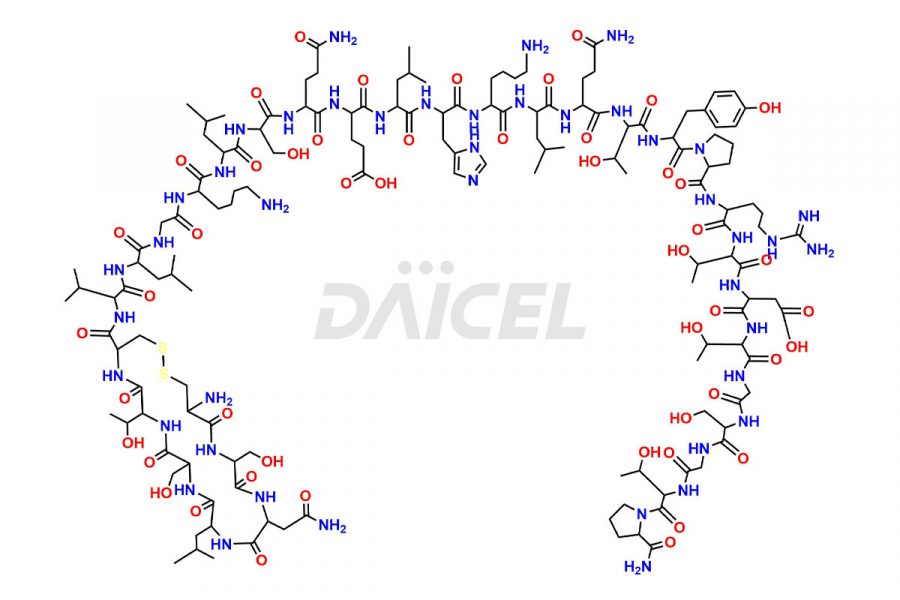
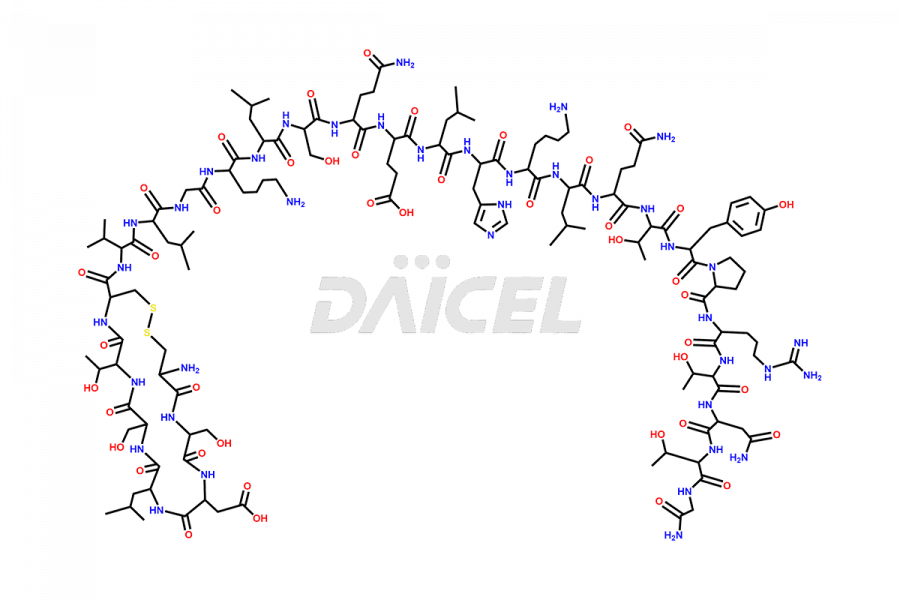
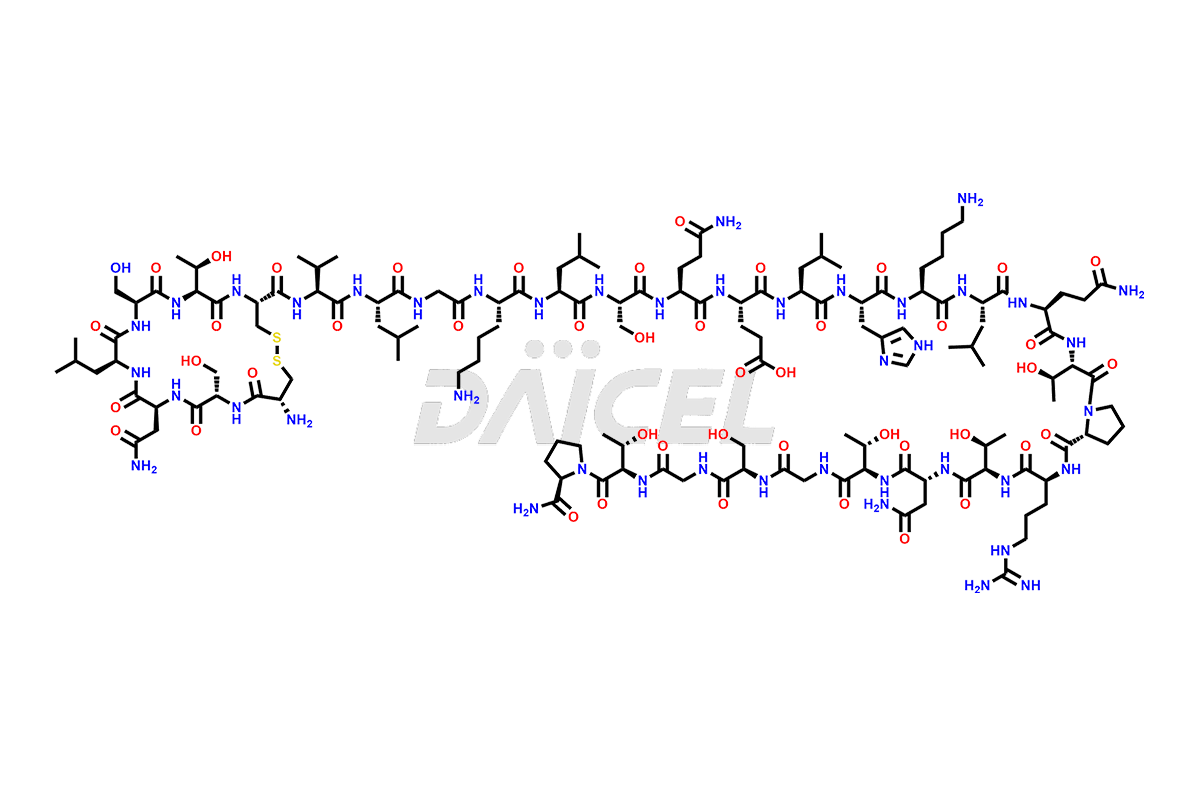
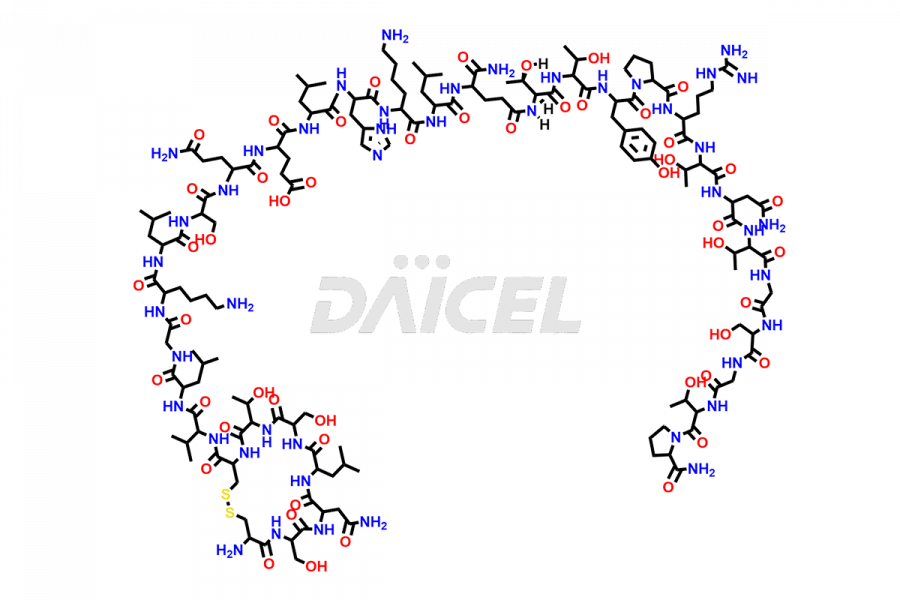
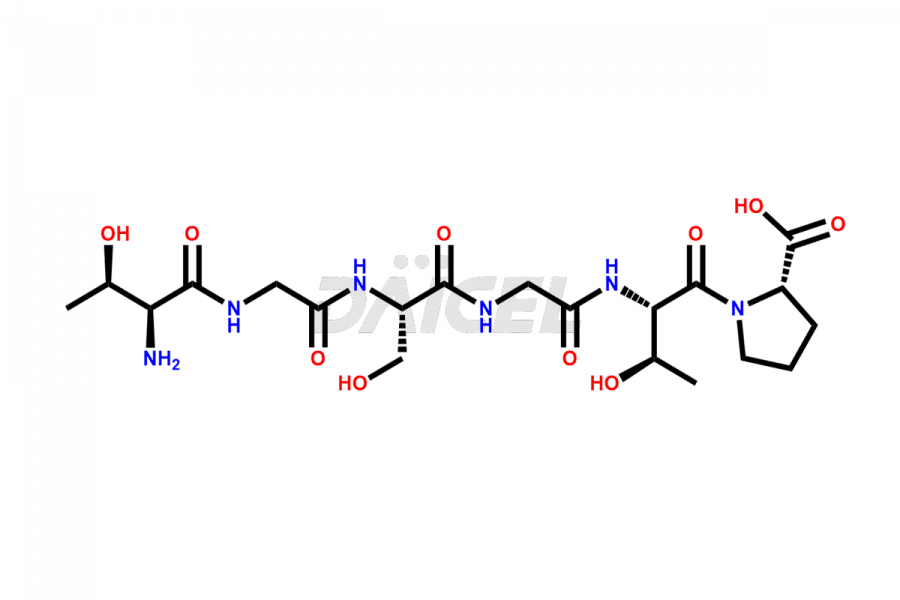
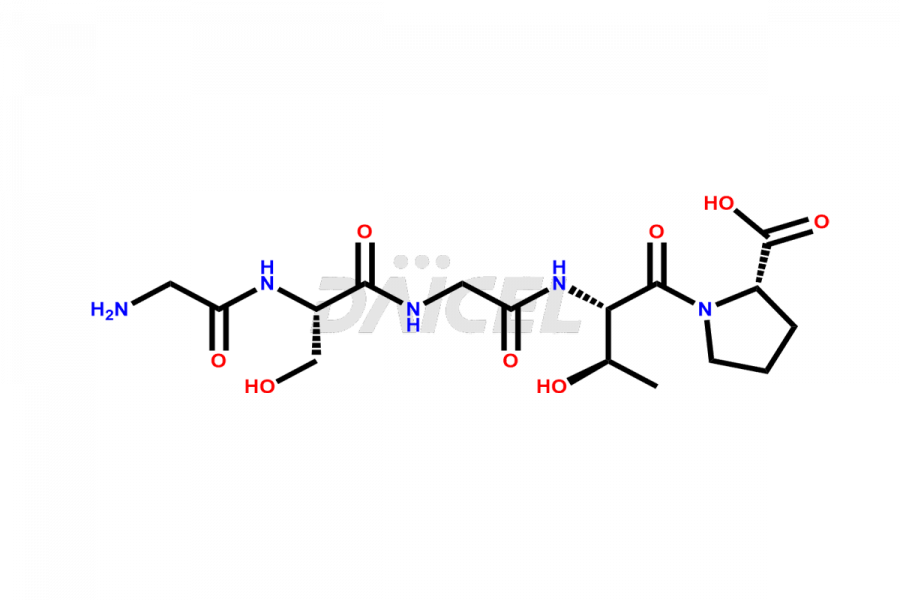
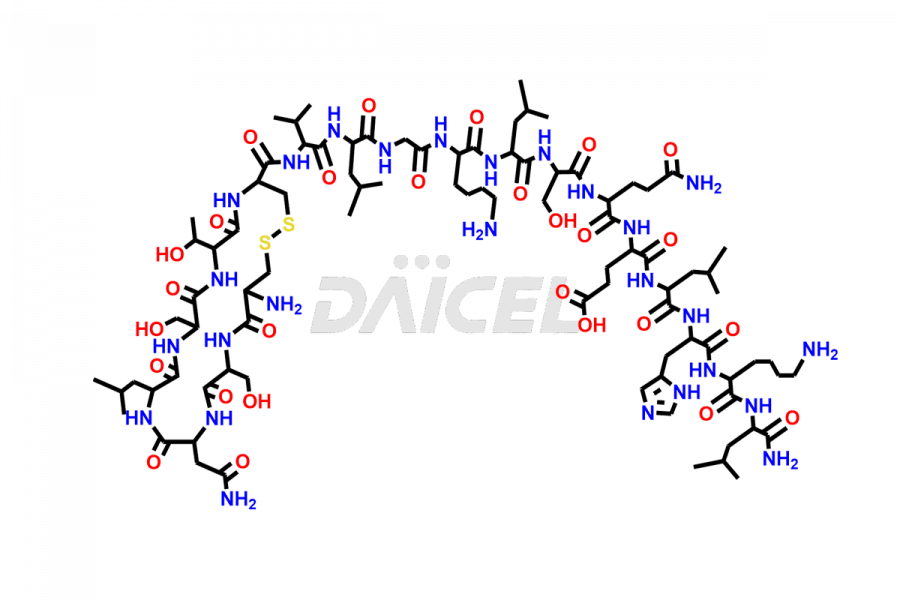
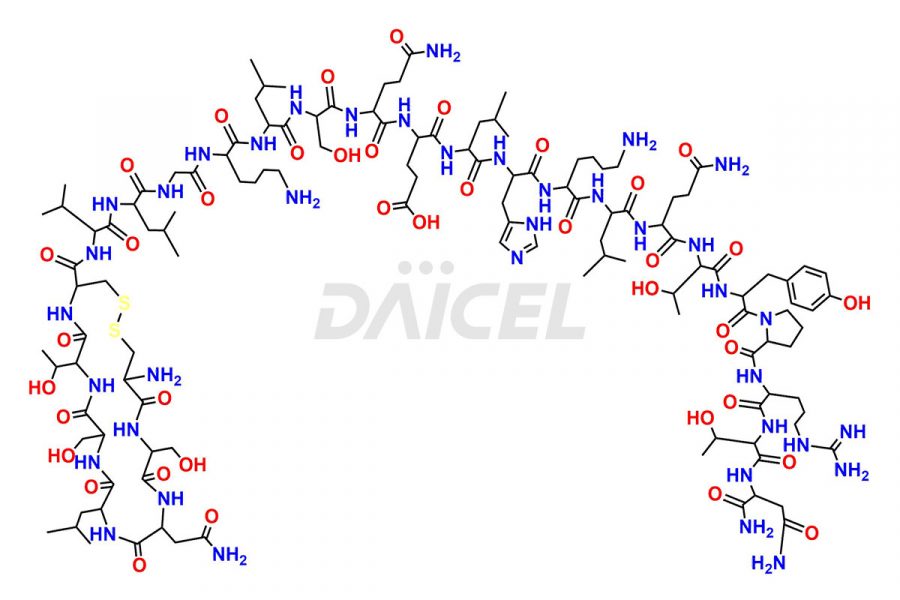
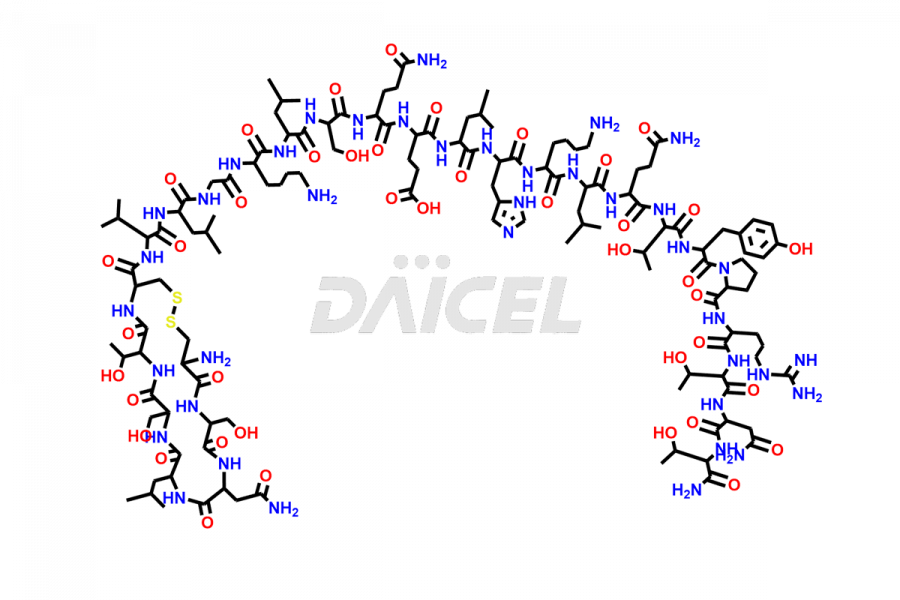
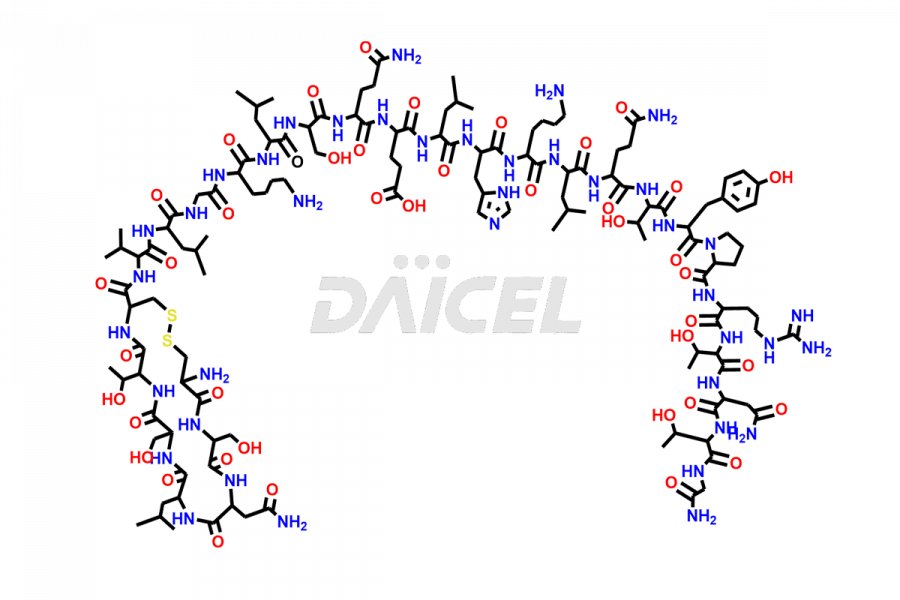
Calcitonin
General Information
Calcitonin Salmon Impurities and Calcitonin Salmon
Daicel Pharma synthesizes more than 20 high-quality Calcitonin Salmon impurities such as 1-2 Hydrolysis-Calcitonin, Asp (3)-Calcitonin, Calcitonin Parallel Dimer, Fragment (27-32)-Calcitonin, Glu-20-Calcitonin, Trisulfide-Calcitonin, Truncated (32-20)-Calcitonin, and more. The impurities are crucial in determining the quality, stability, and biological safety of the active pharmaceutical ingredient, Calcitonin Salmon. Moreover, Daicel Pharma offers custom synthesis of Calcitonin Salmon impurities and delivers them globally.
Salcatonin, also known as Calcitonin Salmon [CAS: 47931-85-1] or Salmon Calcitonin, is a synthetic peptide consisting of 32 amino acids. It has an identical sequence similar to the calcitonin of salmon fish. It is a polypeptide hormone secreted by the thyroid gland’s parafollicular cells. However, compared to human calcitonin, it has 13 different amino acids. Calcitonin Salmon is a medicine authorized for treating symptomatic Paget’s bone disease, hypercalcemia, and osteoporosis in postmenopausal women.
Calcitonin: Use and Commercial Availability
Calcitonin Salmon is commercially available as a nasal spray for a postmenopausal woman having osteoporosis longer than five years and low bone mass. Synthetic Calcitonin Salmon injections treat symptomatic Paget’s bone disease, hypercalcemia, and osteoporosis in postmenopausal women. Calcitonin Salmon maintains or increases bone mass and decreases calcium levels in hypercalcemia. It is sold under the brand names Miacalcin, Calcimar, and Fortical.
Calcitonin Salmon Structure and Mechanism of Action
The chemical formula for Calcitonin Salmon is C145H240N44O48S2, and its molecular weight is approximately 3431.86 g/mol.
The thyroid gland’s parafollicular C cells produce endogenous calcitonin. Calcitonin Salmon is a 32 amino acid alpha-helical polypeptide that differs from human calcitonin due to differences in amino acids 10 to 27. It contracts osteoclasts in bone, reducing their motility and resorption ability. Calcitonin Salmon also inhibits carbonic anhydrase II, which affects the optimal acidic environment for osteoclast activity1.
Calcitonin Salmon Impurities and Synthesis
Calcitonin Salmon may contain impurities such as proteins or peptides from salmon fish, bacterial endotoxins, and residual solvents used in its manufacturing process2. The impurities can lead to various adverse reactions in patients, such as infections, allergies, or other complications. Hence, identifying and measuring structurally related peptide impurities in Calcitonin Salmon is crucial to improve the drug’s quality.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for various Calcitonin Salmon impurities, including 1-2 Hydrolysis-Calcitonin, Asp (3)-Calcitonin, Calcitonin Parallel Dimer, Fragment (27-32)-Calcitonin, Glu-20-Calcitonin, Trisulfide-Calcitonin, Truncated (32-20)-Calcitonin and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC3 purity. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report on delivery.
Daicel has the technology and expertise to prepare any unknown Calcitonin Salmon impurity or degradation product. We also provide labeled compounds to quantify the efficacy of generic Calcitonin Salmon. Daicel offers highly pure Calcitonin Salmon D10 Impurity, a deuterium-labeled standard of Calcitonin Salmon for bioanalytical research and BA/BE studies with isotope data in CoA.
References
FAQ's
References
- Matthew B. McLaughlin; Ishwarlal Jialal, “Calcitonin”, California Northstate University, VA Medical Center, July 18, 2022.
- Guttmann, Stephan; Pless, J.; Huguenin, R. L.; Sandrin, Ed.; Bossert, H.; Zehnder, K., “Synthesis of salmon calcitonin, a highly active hypocalcemic hormone”, Helvetica Chimica Acta, Volume: 52, Issue: 7, Pages: 1789-95,1969.
- Buck, R. H.; Maxl, F., “A validated HPLC assay for salmon calcitonin analysis. Comparison of HPLC and biological assay”, Journal of Pharmaceutical and Biomedical Analysis, Volume: 8, Issue: 8-12, Pages: 761-9, 1990.
Frequently Asked Questions
What are the common types of Calcitonin Salmon impurities?
The most common type of Calcitonin Salmon impurities is other peptides or proteins from Salmon or other fish.
How are Calcitonin Salmon impurities synthesized?
The synthesis of Calcitonin Salmon impurities involves various methods, including solid-phase peptide synthesis (SPPS), recombinant DNA technology, or chemical synthesis.
How can impurities affect the efficacy of Calcitonin Salmon?
Impurities can potentially affect the efficacy of Calcitonin Salmon by interfering with its binding to target receptors or causing adverse reactions in patients.
How are impurities controlled during the synthesis of Calcitonin Salmon?
The control of Impurities involves various steps in the manufacturing process, such as purification and quality control testing.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.