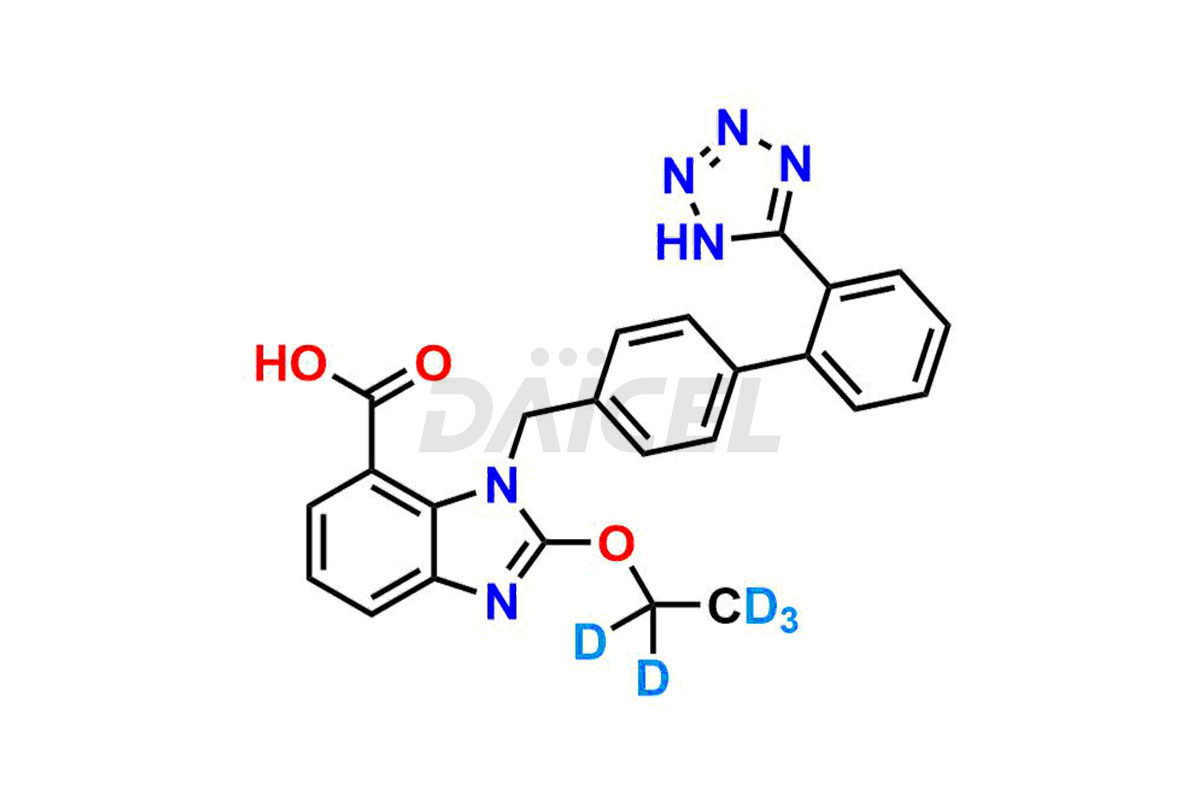
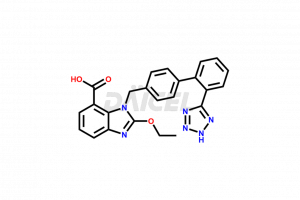
Candesartan
General Information
Candesartan Impurities and Candesartan
Daicel Pharma offers excellent-quality Candesartan impurities, such as Candesartan N2-ethyl Benzimidazole Methyl Ester Impurity. They are vital for evaluating the quality, stability, and biological safety of Candesartan. Furthermore, Daicel Pharma specializes in the custom synthesis of Candesartan impurities and ensures their worldwide delivery.
Candesartan [CAS: 139481-59-7] is a benzimidazole carboxylic acid derivative. It is an angiotensin-receptor blocker that treats hypertension. Candesartan relaxes the blood vessels and prevents the onset of atrial fibrillation. It has a prodrug, candesartan cilexetil.
Candesartan: Use and Commercial Availability
Candesartan is available as Atacand, an oral formulation. It is an antihypertensive agent used alone or with other antihypertensive agents. Further, it treats heart failure in adults with left ventricular systolic dysfunction. Candesartan reduces cardiovascular death. It prevents migraine and manages diabetic renal disease in patients.
Candesartan Structure and Mechanism of Action
The chemical name of Candesartan is 2-Ethoxy-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-benzimidazole-7-carboxylic acid. The chemical formula for Candesartan is C24H20N6O3, and its molecular weight is approximately 440.45 g/mol.
Candesartan selectively blocks the binding of angiotensin II to AT1 receptors in tissues like the adrenal gland and vascular smooth muscles. It prevents the vasoconstrictor properties of angiotensin II receptors.
Candesartan Impurities and Synthesis
During Candesartan synthesis1, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Candesartan. And so, Candesartan impurities must be controlled and monitored throughout the drug’s development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Candesartan impurities, which includes Candesartan N2-ethyl Benzimidazole Methyl Ester Impurity. Daicel Pharma offers CoA from a cGMP-compliant analytical facility. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Candesartan impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Candesartan Labelled Standard. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
FAQ's
References
- Naka, Takehiko; Nishikawa, Kohei; Kato, Takeshi, Benzimidazole derivatives, their production and use, EP459136B1, Dec 27, 1996, Takeda Chemical Industries, Ltd., Japan (https://patents.google.com/patent/EP0459136A1/en)
- Stenhoff, Helene; Lagerstrom, Per-Olof; Andersen, Cathrine, Determination of candesartan cilexetil, candesartan and a metabolite in human plasma and urine by liquid chromatography and fluorometric detection, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 731, Issue: 2, Pages: 411-417, 1999 DOI: (10.1016/s0378-4347(99)00247-9)
Frequently Asked Questions
2. What are the analytical methods to identify and characterize Candesartan impurities?
Semi-preparative HPLC and NMR spectroscopy are the analytical methods that help identify and characterize Candesartan impurities.
3. Which analytical method separates the degradation products and process-related impurities of Candesartan from the drug product?
The ultra-performance liquid chromatography is the analytical method that separates the degradation products and process-related impurities of Candesartan from the drug product.
4. Why is it necessary to eliminate nitrosamine impurities from Candesartan drug substance?
Nitrosamine impurities are carcinogenic and affect drug safety. Hence, it is necessary to eliminate nitrosamine impurities from the Candesartan drug substance.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.