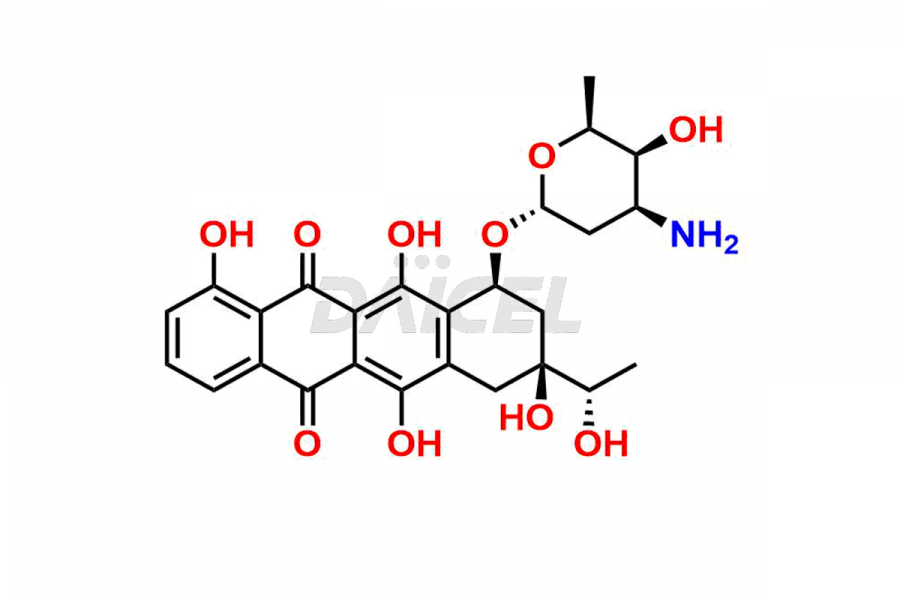
Carminomycin
General Information
Carminomycin Impurities and Carminomycin
Daicel Pharma is a reliable partner in synthesizing the best-quality Carminomycin impurities, such as 13-(S) Dihydrocarminomycin. It is vital to evaluate Carminomycin quality, stability, and biological safety. Further, Daicel Pharma is an expert in custom synthesizing Carminomycin impurities and ensuring their delivery globally.
Carminomycin [CAS: 39472-31-6] is an antineoplastic agent. It is an anthracycline antibiotic produced by Actinomadura carminata1. It is an analog of Daunomycin that treats adults with acute myeloid leukemia.
Carminomycin: Use and Commercial Availability
Carminomycin is an antimicrobial agent that works against microorganisms like bacteria, viruses, fungi, etc. It kills cancer cells and prevents neoplasm proliferation. Its antitumor properties are effective against different types of cancer. Carminomycin is available under various brands such as Karminomitsin, Karminomycin, Antibiotic R 588A, etc.
Carminomycin Mechanism of Action
The chemical name of Carminomycin is 9-acetyl-7-[4-amino-5-[3-hydroxy-1-(1-hydroxypropan-2-yloxy)butoxy]-6-methyloxan-2-yl]oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7H-tetracene-5,12-dione. Its chemical formula is C33H41NO13.
Carminomycin selectively blocks nucleic acid synthesis in cells of microorganisms and malignant tumors. It prevents repair in bacterial cells affected by radiation and alklylating agents. It inhibits DNA Topoisomerase II activity that blocks DNA replication with resultant apoptosis.
Carminomycin Impurities and Synthesis
During Carminomycin synthesis, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Carminomycin. It is necessary to control and monitor Carminomycin impurities throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Carminomycin impurities, which includes 13-(S) Dihydrocarminomycin. The CoA is from a cGMP-compliant analytical facility. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Carminomycin impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Carminomycin for bioanalytical research and BA/BE studies. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
- https://link.springer.com/chapter/10.1007/978-1-4613-4352-3_29
- Fandrich, Susan E.; Pittman, Kenneth A, Analysis of Carminomycin in human serum by fluorometric high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 223, Issue: 1, Pages: 155-64, 1981 DOI: (10.1016/s0378-4347(00)80078-x)
Frequently Asked Questions
2. How do Carminomycin impurities form in the drug substance?
Unreacted raw materials, intermediates, by-products, residual solvents, reagents, and catalysts form Carminomycin impurities in the drug.
3. Why do we need to control Carminomycin impurities in the drug substance?
The presence of Carminomycin impurities in the drug substance will affect the drug’s safety and quality. Hence, it is essential to remove them from the drug substance.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.