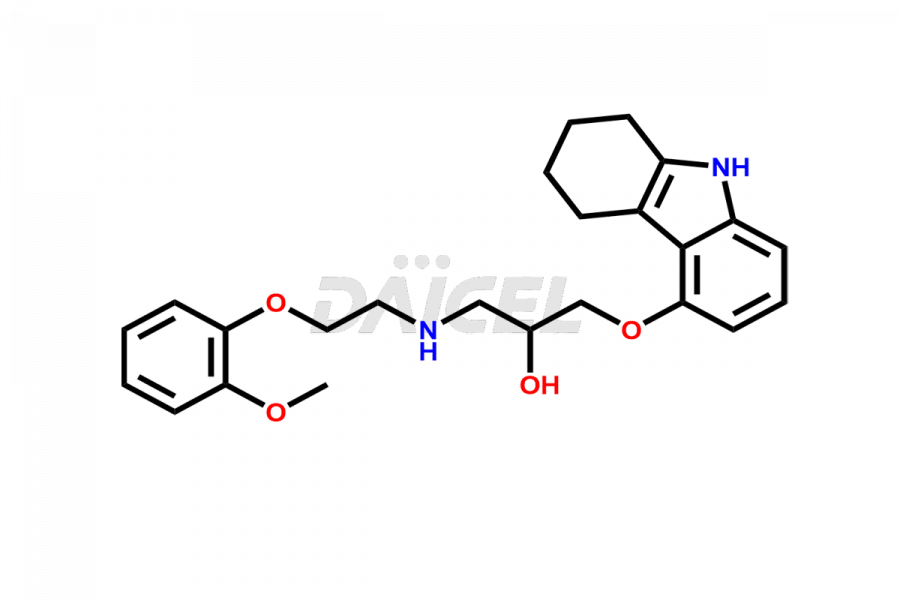
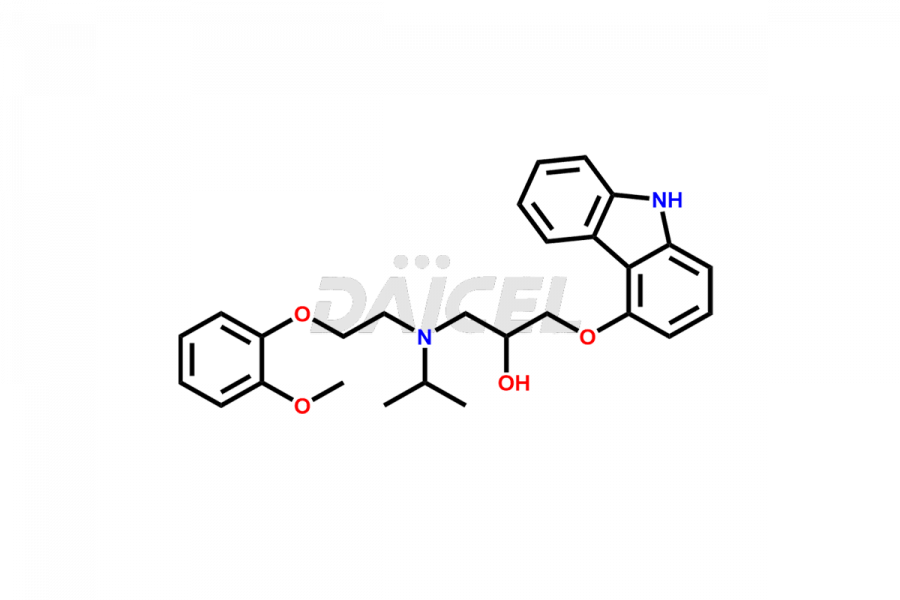
Carvedilol
References
- Wiedemann, Fritz; Kampe, Wolfgang; Thiel, Max; Sponer, Gisbert; Roesch, Egon; Dietmann, Karl, Derivatives Of Carbazolyl-4-Oxypropanolamine, Methods For Their Preparation And Medicines Containing Them, Boehringer Mannheim G.m.b.H., Federal Republic of Germany, EP4920B1, August 5, 1981
- Reiff, K., High-performance liquid chromatographic method for the determination of carvedilol and its demethyl metabolite in body fluids, Journal of Chromatography, Biomedical Applications, Volume: 413, Pages: 355-62, 1987
Frequently Asked Questions
How are Carvedilol impurities identified?
Impurities in Carvedilol are identified using analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography (LC), mass spectrometry (MS), etc.
What are the limits for Carvedilol impurities set by regulatory agencies?
The limits for impurities in Carvedilol set by regulatory agencies are ≤0.2% for individuals and ≤0.5% for total impurities.
What is the role of impurity standards in Carvedilol?
Impurity standards in Carvedilol help identify and quantify impurities in the drug substance. They are also used to develop and validate analytical methods for impurity identification and quantification.
What are the temperature conditions required to store Carvedilol impurities?
Carvedilol impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.