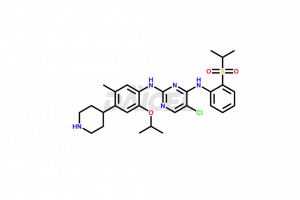
Ceritinib
General Information
Ceritinib Impurities and Ceritinib
Daicel Pharma offers Ceritinib impurities of the best quality, including 1-chloro-5-isopropoxy-2-methyl-3-nitrobenzene. The impurities can impact the efficacy, stability, and safety of Ceritinib. Daicel Pharma can synthesize custom Ceritinib impurities and deliver them worldwide.
Ceritinib [CAS: 1032900-25-6] is an aminopyrimidine compound that treats adults with metastatic non-small cell lung cancer (NSCLC). It is an anaplastic lymphoma kinase (ALK) inhibitor and prevents carcinogenesis. Further, Ceritinib is a Cytochrome P450 3A inhibitor.
Ceritinib: Use and Commercial Availability
As an anti-cancer agent, Ceritinib treats adults with non-small cell lung cancer (NSCLC), which is ALK-positive and has spread to other parts of the body. It helps in cancer therapy in patients who had a failure with prior Crizotinib therapy. Ceritinib also treats various types of cancer. Ceritinib is available as an oral medication under Zykadia.
Ceritinib Structure and Mechanism of Action
The chemical name of Ceritinib is 5-Chloro-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-N2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine. Its chemical formula is C28H36ClN5O3S, and its molecular weight is approximately 558.14 g/mol.
Ceritinib inhibits autophosphorylation of anaplastic lymphoma kinase (ALK). It blocks ALK-mediated phosphorylation of STAT3, the downstream signaling protein. Thus, it prevents the spread of ALK-dependent cancer cells.
Ceritinib Impurities and Synthesis
Impurities can occur during the synthesis1 of Ceritinib by mechanisms, including incomplete reactions, side reactions, and the contaminants in the starting materials or reagents. Strict quality control measures are needed while preparing Ceritinib to avoid unwanted side effects caused by these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Ceritinib impurities, which include 1-chloro-5-isopropoxy-2-methyl-3-nitrobenzene. Our CoA is from our cGMP-certified analytical laboratory and includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We provide further characterization details upon request. Our team of professionals at Daicel Pharma specializes in providing Ceritinib impurities and degradation compounds. Daicel Pharma provides highly purified, stable isotope-labeled standards of Ceritinib.
References
References:
- Michellys, Pierre-Yves; Pei, Wei; Marsilje, Thomas H.; Lu, Wenshuo; Chen, Bei; Uno, Tetsuo; Jin, Yunho; Jiang, Tao, Compounds and compositions as protein kinase inhibitors, WO2008073687A2, Jun 19, 2008, IRM LLC, Bermuda (https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2008073687)
- Heudi, Olivier; Vogel, Denise; Lau, Yvonne Y.; Picard, Franck; Kretz, Olivier, Liquid chromatography tandem mass spectrometry method for the quantitative analysis of ceritinib in human plasma and its application to pharmacokinetic studies, Analytical and Bioanalytical Chemistry, Volume: 406, Issue: 28, Pages: 7389-7396, 2014 DOI: (10.1007/s00216-014-8125-9)
Frequently Asked Questions
2. Why should Ceritinib genotoxic impurities be removed from the drug product?
Genotoxic impurities are carcinogenic and can potentially interact with the human DNA. Hence, it is necessary to remove Ceritinib genotoxic impurities from the drug product.
3. How do Ceritinib impurities form in the drug substance?
Raw materials, reagents, and unwanted side reactions form Ceritinib impurities in the drug.
4. Why is there a need to control Ceritinib nitrosamine impurities in the drug substance?
Ceritinib nitrosamine impurities in the drug substance can affect the drug’s safety and quality. Hence, it is essential to remove them from the drug substance.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.