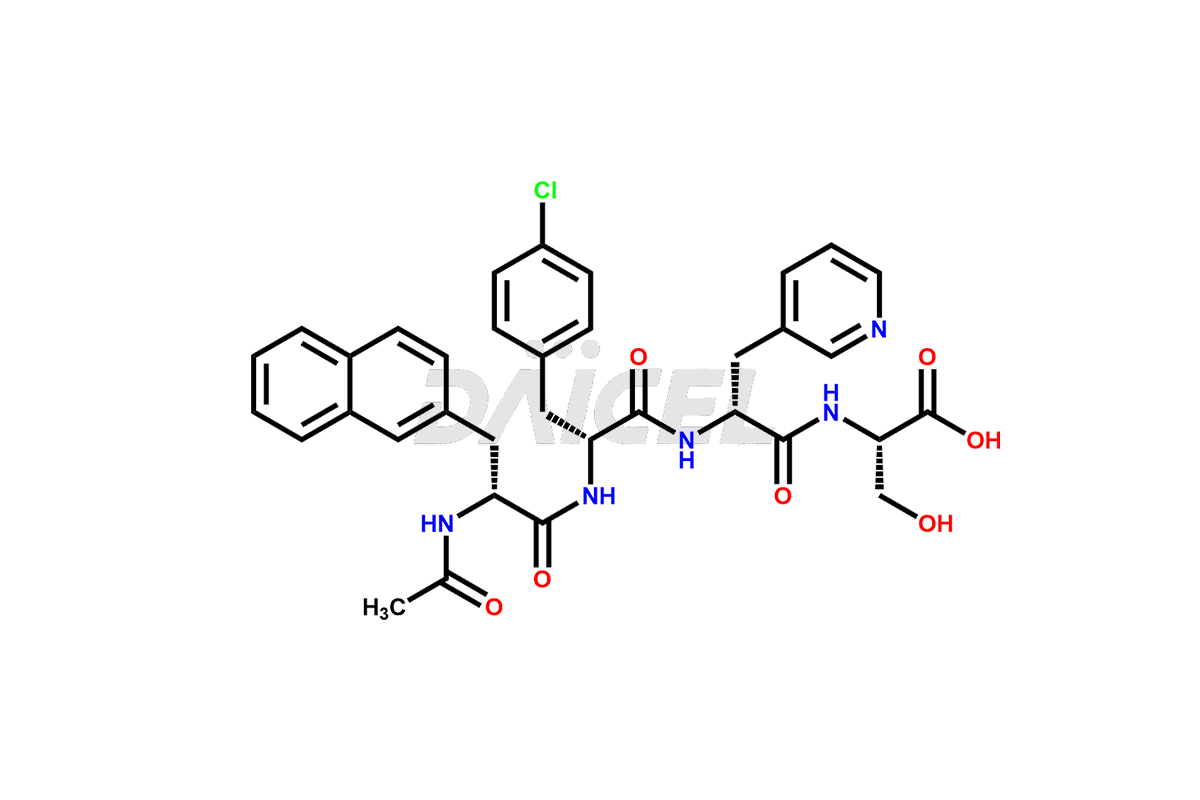
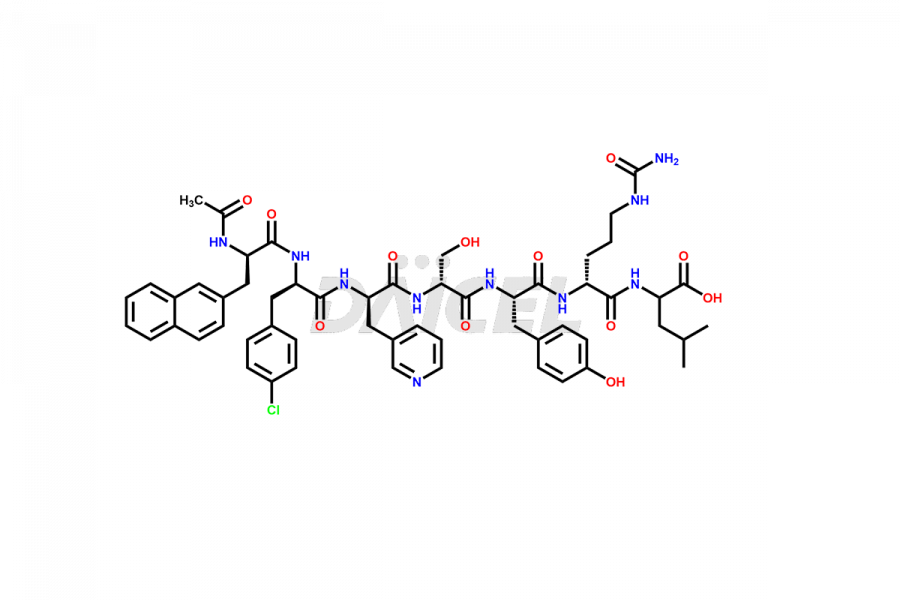
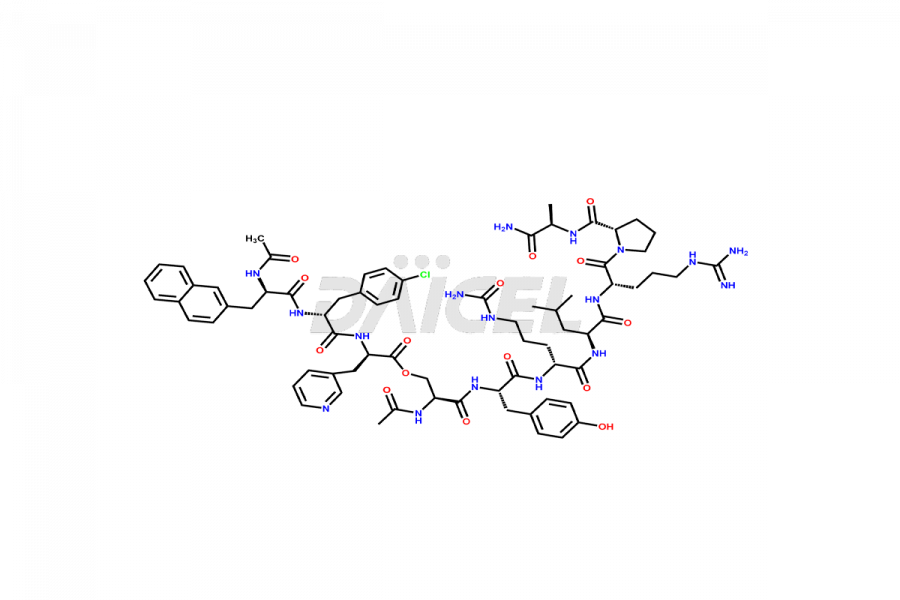
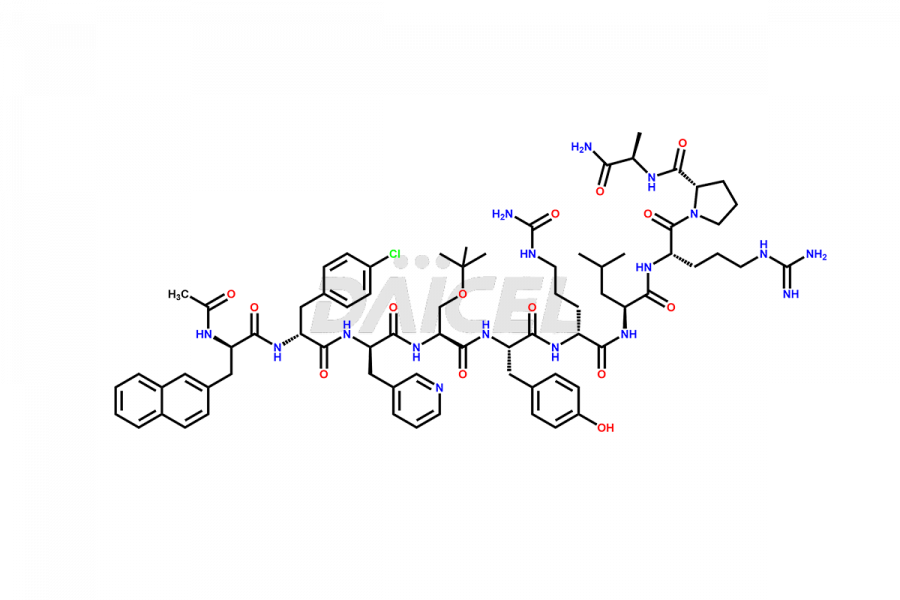
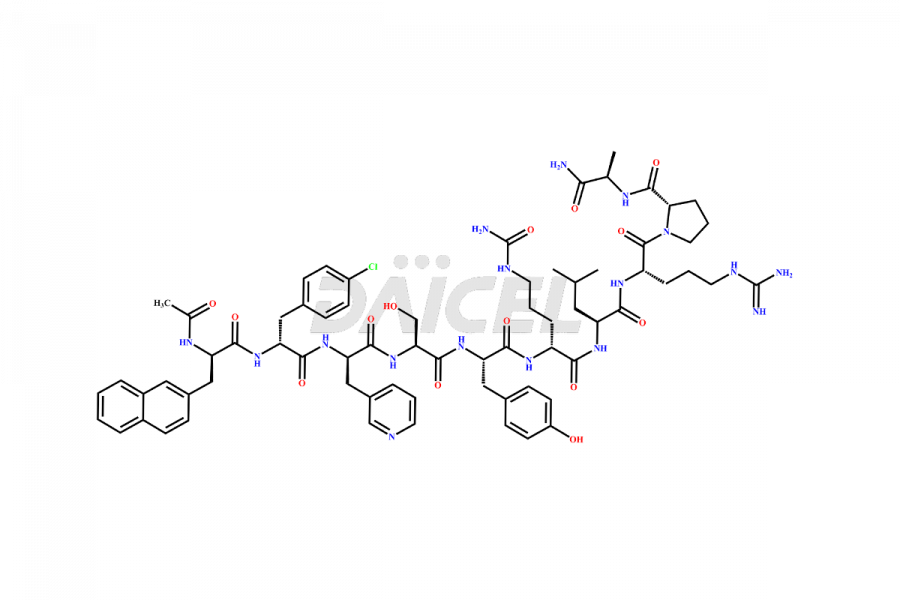
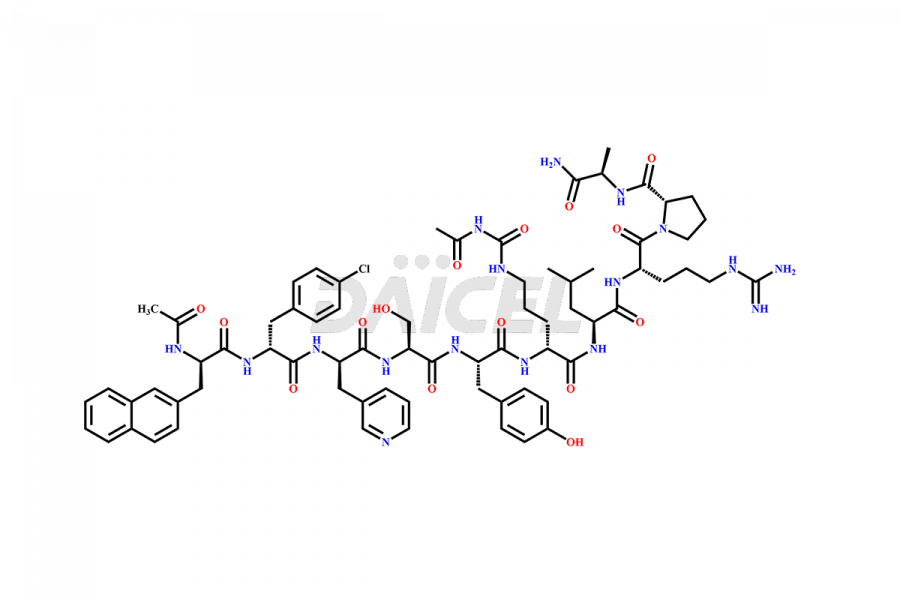
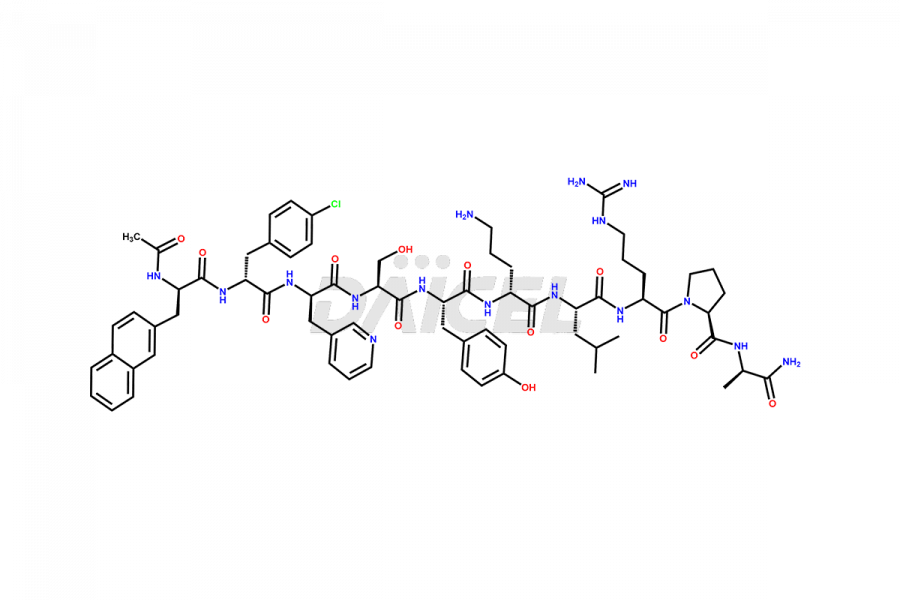
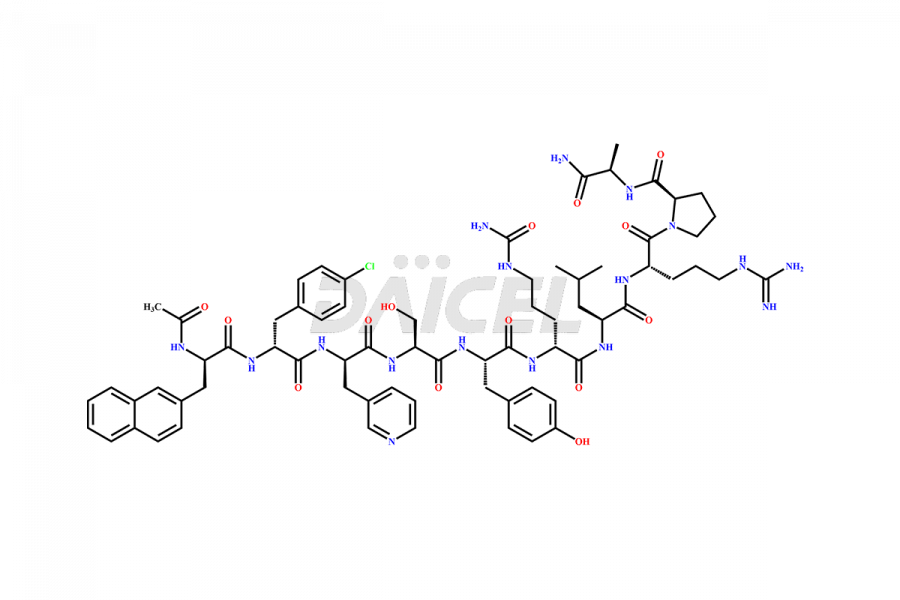
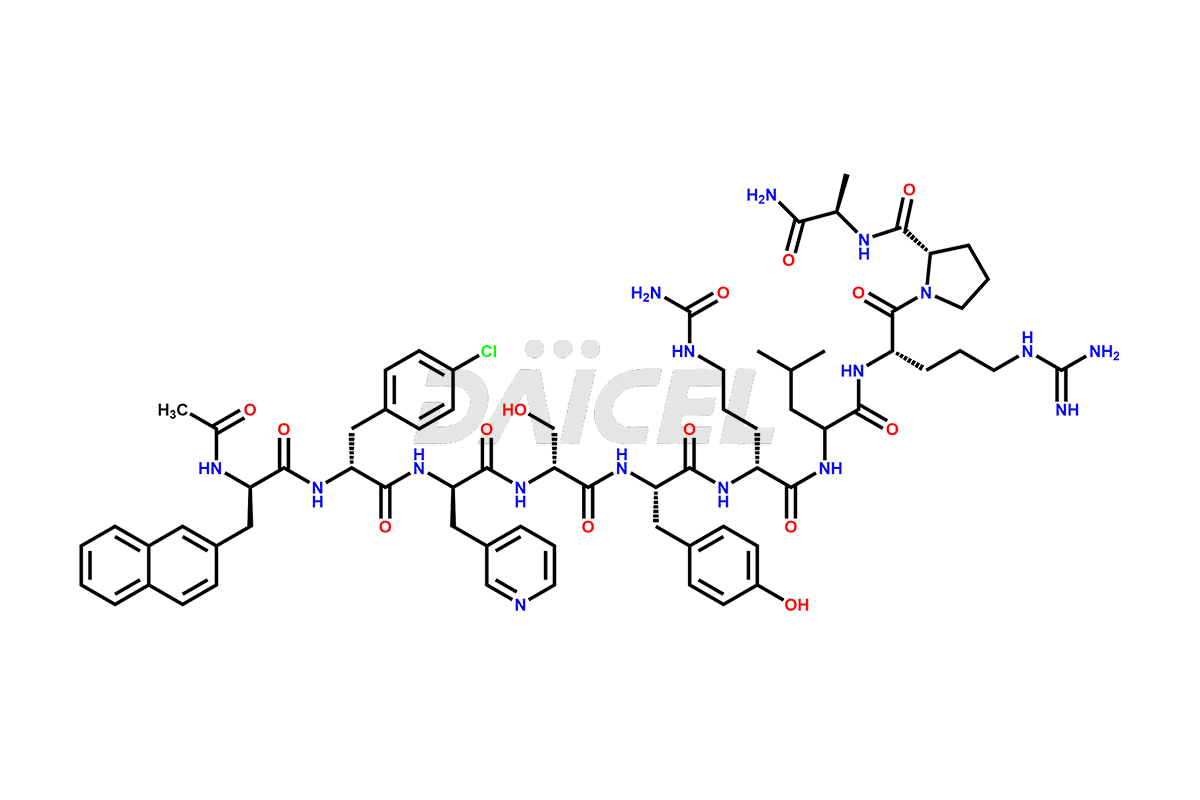
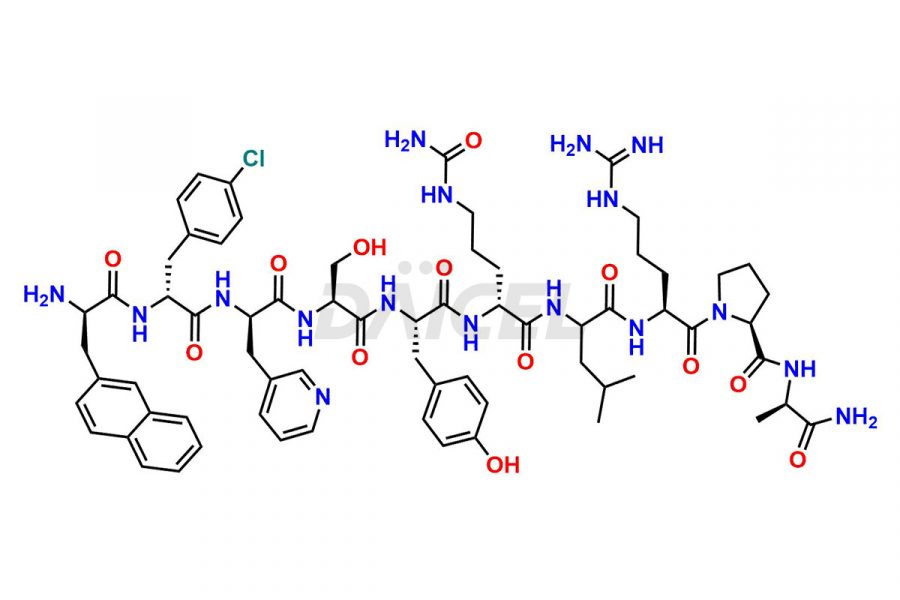
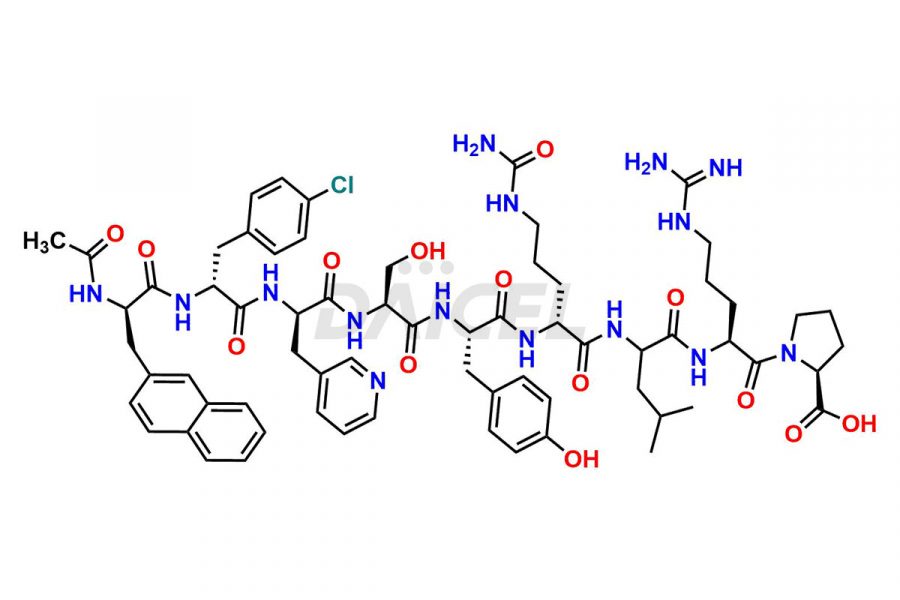
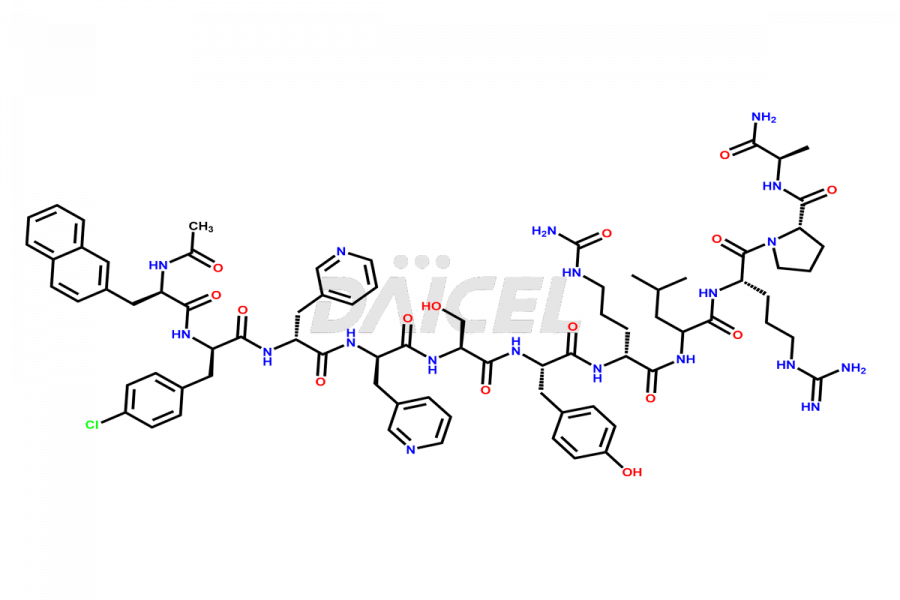
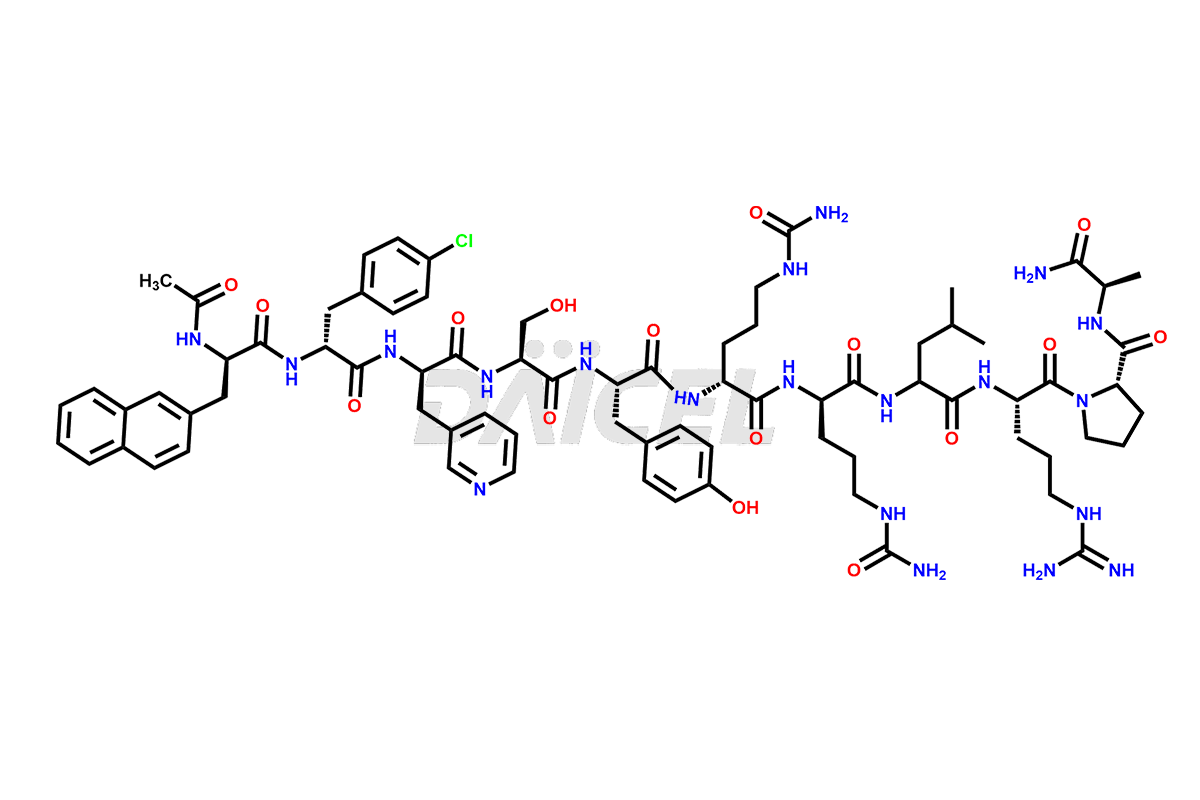
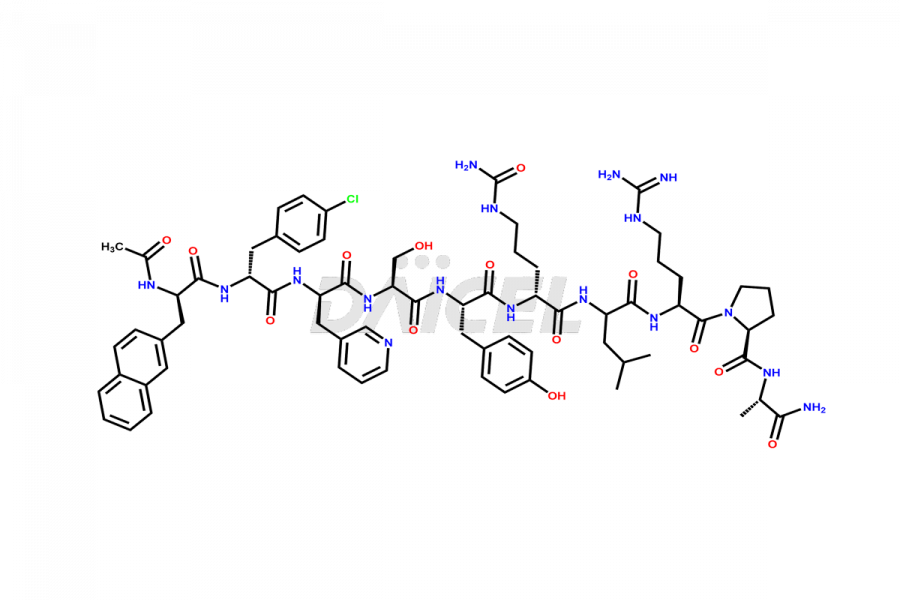
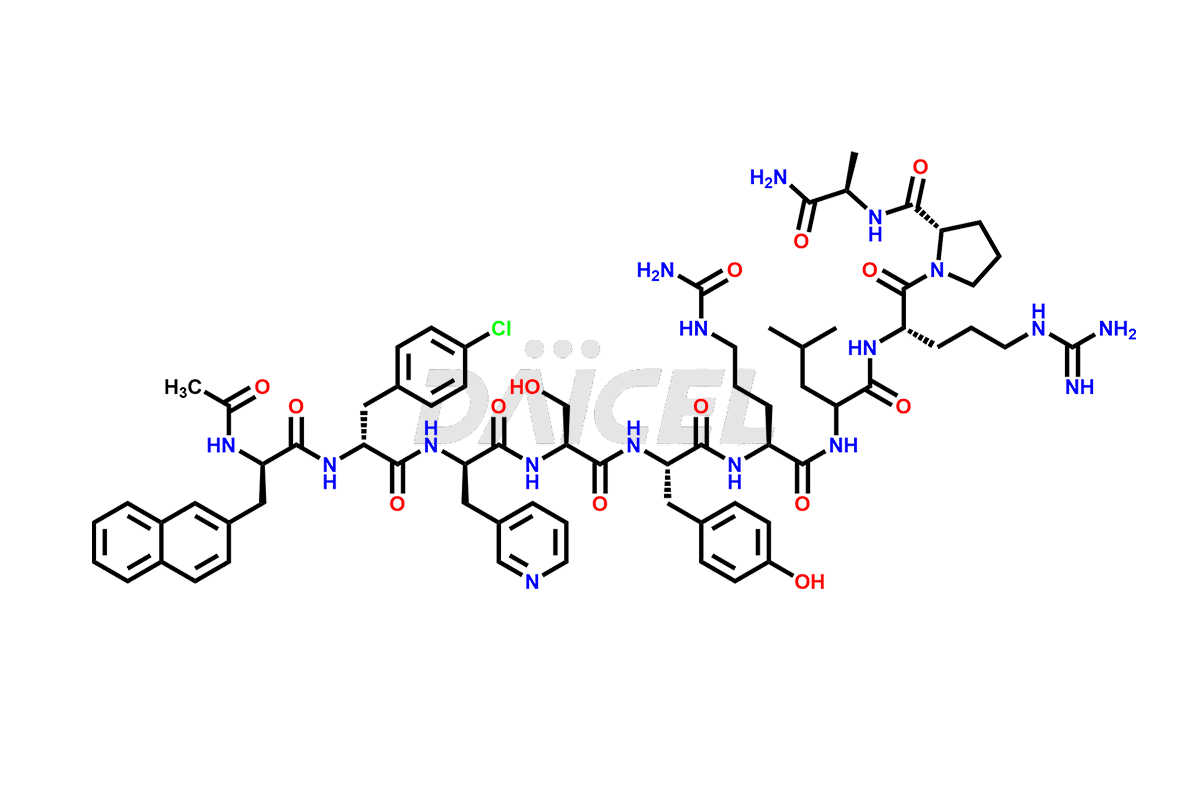
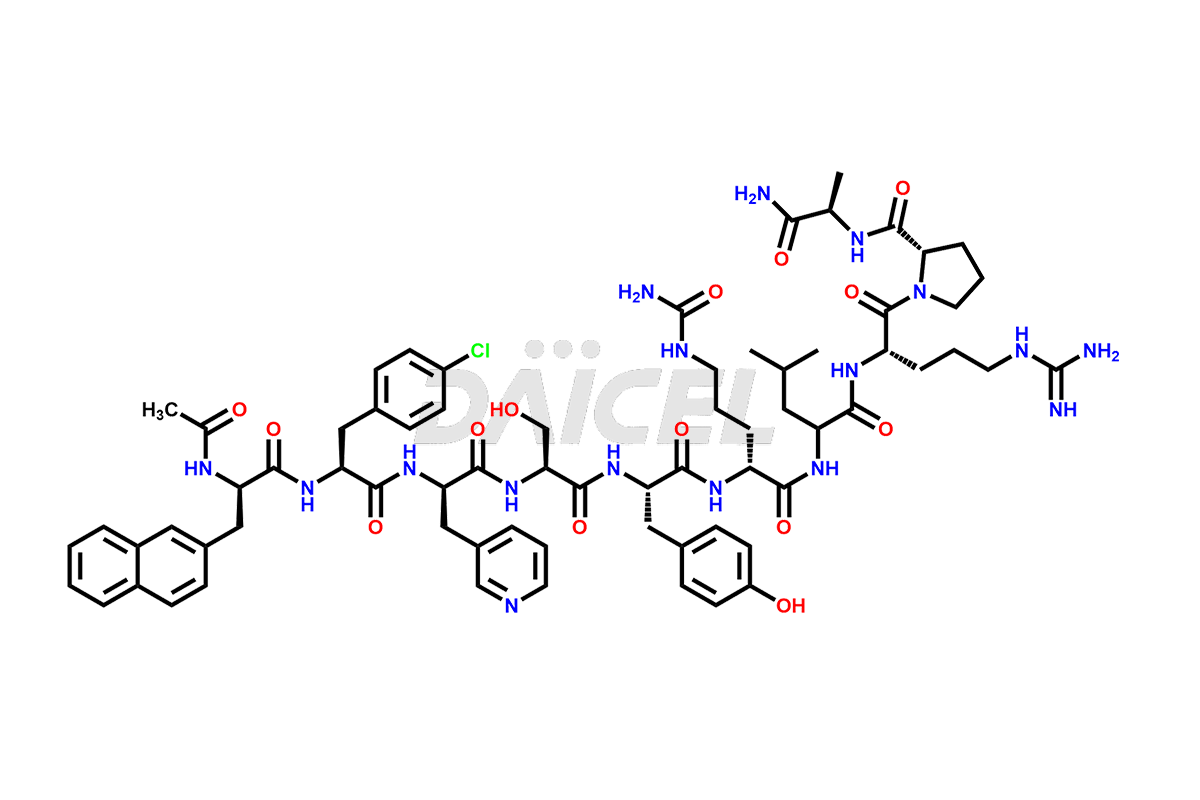
Cetrorelix
General Information
Cetrorelix Impurities and Cetrorelix
Daicel Pharma synthesizes high-quality Cetrorelix impurities such as Cetrorelix dimer, and more, that contribute to the quality, stability, and biosafety analysis of Cetrorelix.
Cetrorelix [CAS: 120287-85-6] is a synthetic decapeptide gonadotropin-releasing hormone (GnRH) antagonist involved in the release of luteinizing hormone (LH). It prevents the premature surge of luteinizing hormone in women undergoing assisted reproductive therapies. The presence of Cetrorelix impurities produced during the drug manufacturing process may compromise the safety and efficacy of the drug. Hence, it is vital to ensure the purity of Cetrorelix.
Cetrorelix: Use and Commercial Availability
Cetrorelix is a drug used primarily in assisted reproductive technology (ART) to prevent premature ovulation in women undergoing controlled ovarian stimulation. It is a gonadotropin-releasing hormone (GnRH) antagonist that blocks the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which stimulates ovulation. Cetrorelix is under development for treating prostate cancer, ovarian cancer, and benign prostatic hyperplasia.
The administration of Cetrorelix involves an injection under the skin of the lower abdomen. Its use is for a short period during treatment. It may be available in vials or pre-filled syringes. Cetrotide and Cetrolix are the brand names under which Cetrorelix is sold.
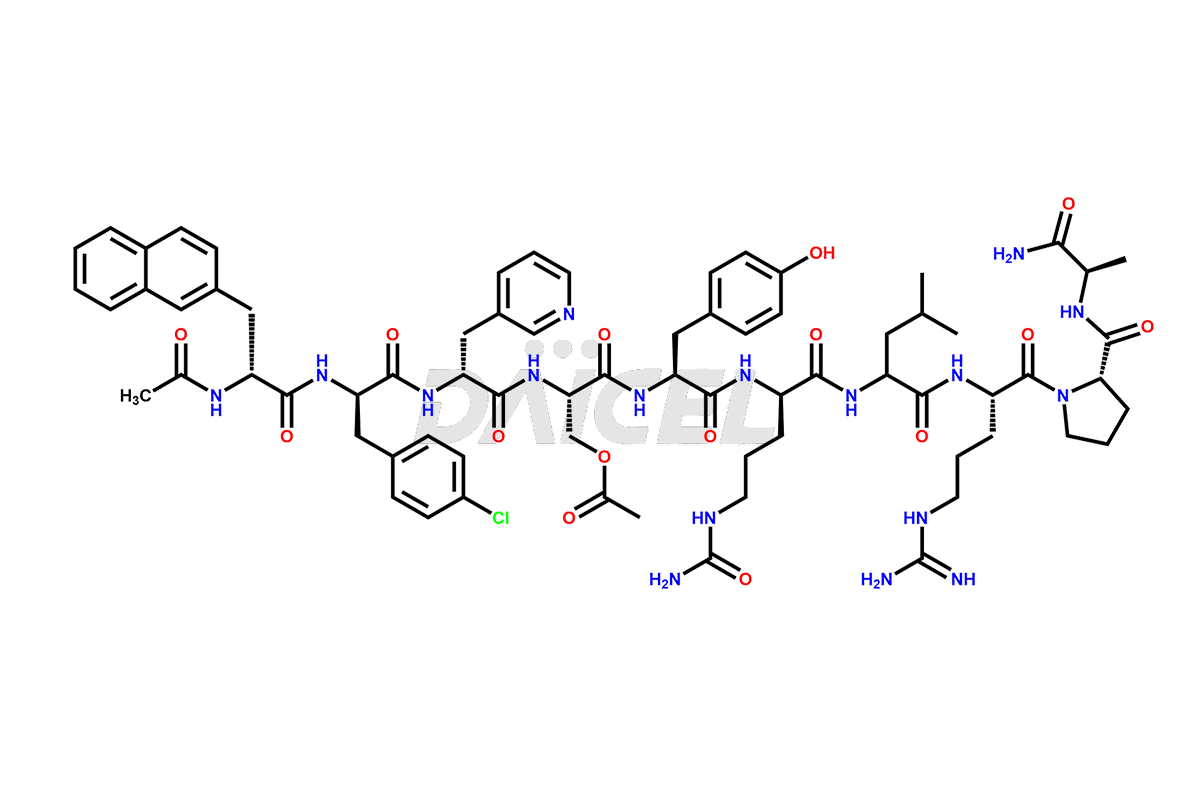
Cetrorelix Structure and Mechanism of Action
The chemical formula of Cetrorelix is C70H92ClN17O14, and its molecular weight is 1431.038 g/mol.
Cetrorelix binds to gonadotropin-releasing hormone receptors and acts as a potent inhibitor of gonadotropin secretion. It competes with natural GnRH for binding to pituitary cell membrane receptors, thereby controlling LH and FSH release in a dose-dependent manner.
This leads to a suppression of the production of estrogen, the chief hormone responsible for the growth and development of the endometrium in preparation for the implantation of a fertilized egg.
Cetrorelix Impurities and Synthesis
Impurities such as des-acetyl, oxidized, deamidated, and truncated peptides may be present in the manufacturing process of Cetrorelix1. It is important to control and monitor impurities in the Cetrorelix manufacturing process to ensure the quality of the final product.
At Daicel, we offer Cetrorelix impurity standards such as Cetrorelix Dimer, Des-Acetyl-Cetrorelix, Des-Amido-Cetrorelix, Des-D-Ala-Cetrorelix, Endo-3-D-Pal-Cetrorelix, and L-Ala(10)-Cetrorelix. We also offer Cetrorelix-d10, which is a deuterium labeled Cetrorelix standard used in bio-analytical research such as BA/BE studies with isotope data in CoA. Further, we have the technology and expertise to prepare any unknown Cetrorelix impurity or degradation product.
We provide a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for all Cetrorelix impurities with complete characterization data like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We provide 13C-DEPT and CHN on request. Also, we provide a complete characterization report upon delivery.
References
FAQ's
References
- Andrew V. Schally; Sandor Bajusz, Asta Pharma A.-G., Federal Republic of Germany, “LHRH antagonists” US patent US4800191, Jan 24, 1989
- Manik Reddy Pullagurla, Jagadeesh Babu Rangisetty, Biophore India Pharmaceuticals Private Limited, “Process for the preparation of cetrorelix acetate” US 2019/0382447 Al, Dec.9,2019
Frequently Asked Questions
Why is the synthesis of Cetrorelix impurities essential?
The synthesis of Cetrorelix impurities is essential for the quality control of the drug substance and product. The identification and characterization of impurities help to ensure that the drug is safe and effective for use in patients.
How are Cetrorelix impurities synthesized?
Cetrorelix impurities synthesis involves using the solid-phase peptide synthesis (SPPS) method. The peptide chain is built up by sequentially adding protected amino acid residues, activated by coupling agents, on a solid support resin. After assembling the peptide chain, the protecting groups are removed, and the peptide is cleaved from the resin and purified by chromatography techniques such as RP-HPLC.
How are Cetrorelix impurities detected and quantified?
Cetrorelix impurities are detected and quantified using analytical methods such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
At what temperature should Cetrorelix impurities be stored?
The storage temperature for Cetrorelix impurities may depend on the specific type of impurities and the manufacturer's recommendations. Cetrorelix impurities are stored in a cool, dry place, between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.