Cibenzoline
General Information
Cibenzoline Impurities and Cibenzoline
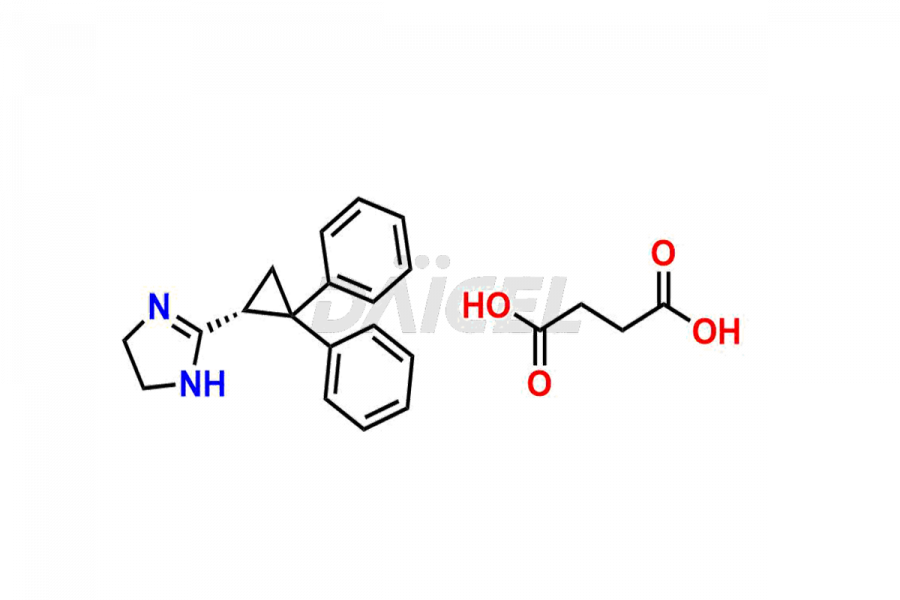
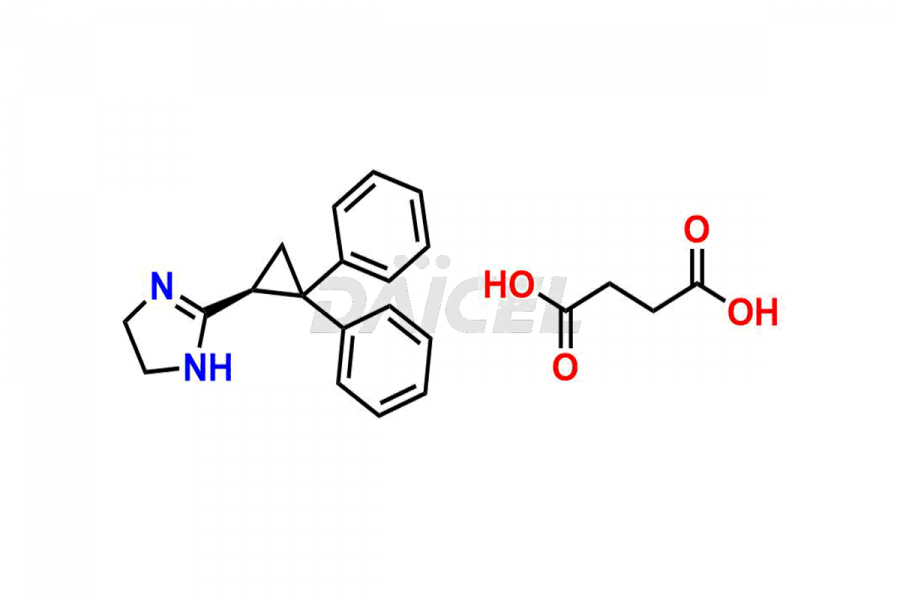
Daicel Pharma synthesizes Cibenzoline impurities of exceptional quality, such as (R)-(+)-Cibenzoline succinate and (S)-(-)-Cibenzoline succinate. These impurities are crucial to assess the purity, reliability, and safety of Cibenzoline, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Cibenzoline impurities to meet clients’ demands for delivery worldwide.
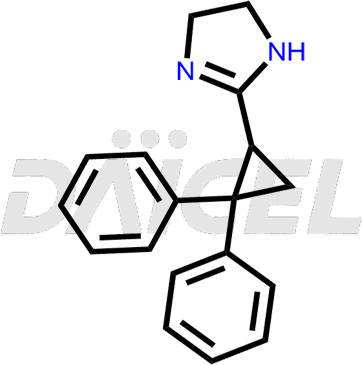
Cibenzoline [CAS: 53267-01-9], known for its favorable pharmacokinetic characteristics, demonstrates notable effectiveness as an antiarrhythmic agent. It can be included in the treatment regimen alongside other class I medications, particularly for high-risk arrhythmias patients.
Cibenzoline: Use and Commercial Availability
Cibenzoline belongs to class I of antiarrhythmic drugs. Its administration can be either orally or intravenously. Cibenzoline treats both ventricular and supraventricular arrhythmias, including those that are resistant to other medications, particularly in cases of ventricular tachycardia or arrhythmias following recent acute myocardial infarction. Cibenzoline is available under brand names such as Cifenline, Exacor, and Cipralan.
Cibenzoline Structure and Mechanism of Action 
The chemical name of Cibenzoline is 2-(2,2-Diphenylcyclopropyl)-4,5-dihydro-1H-imidazole. Its chemical formula is C18H18N2, and its molecular weight is approximately 262.3 g/mol.
The mechanism of action of Cibenzoline is unknown.
Cibenzoline Impurities and Synthesis
The impurities in Cibenzoline, an antiarrhythmic drug, can occur during its synthesis1, storage, or degradation. These impurities may arise from incomplete reactions, side reactions, or degradation of the active ingredient. It is crucial to analyze and control these impurities due to their potential impact on the drug’s safety, efficacy, and quality. Impurity analysis helps to identify and quantify these substances, ensuring compliance with regulatory guidelines and pharmacopeial standards. By implementing strict control measures, such as setting acceptable limits for impurity levels, manufacturers can maintain the purity and quality of Cibenzoline, thereby ensuring its safety and effectiveness in clinical use.
Daicel Pharma offers a Certificate of Analysis (CoA) for Cibenzoline impurity standards, such as (R)-(+)-Cibenzoline succinate and (S)-(-)-Cibenzoline succinate generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Cibenzoline impurities or degradation products and labeled compounds to assess the effectiveness of Cibenzoline. We also offer (S)-(-)-Cibenzoline-D4 and R-(+)-Cibenzoline-D4, deuterium-labeled Cibenzoline compounds useful in bio-analytical research, such as BA/BE studies. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Jean-Claude Cognaco, Chlorinated derivatives of 1-(2-{66 {hu 2{b -imidazolinyl)-2,2-diarylcyclopropanes, Societe Anonyme dite: Hexachime, Rueil Malmaison, France, US3905993A, September 16, 1975
- Hackman, Martin R.; Lee, Teh Lo; Brooks, Marvin A., Determination of cibenzoline in plasma and urine by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 273, Issue: 2, Pages: 347-56,1983
Frequently Asked Questions
How is the analysis of Cibenzoline impurities performed?
The analysis involves various techniques, such as chromatography (HPLC, LC), spectroscopy (UV, IR), and mass spectrometry.
What are the measures if an impurity exceeds the established limits in Cibenzoline?
If an impurity exceeds the limits, appropriate corrective measures like process optimization, formulation adjustments, or purification techniques are implemented.
Which solvent helps in the analysis of Cibenzoline impurities?
Methanol is a solvent used in analyzing many impurities in Cibenzoline.
What are the temperature conditions required to store Cibenzoline impurities?
Cibenzoline impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.