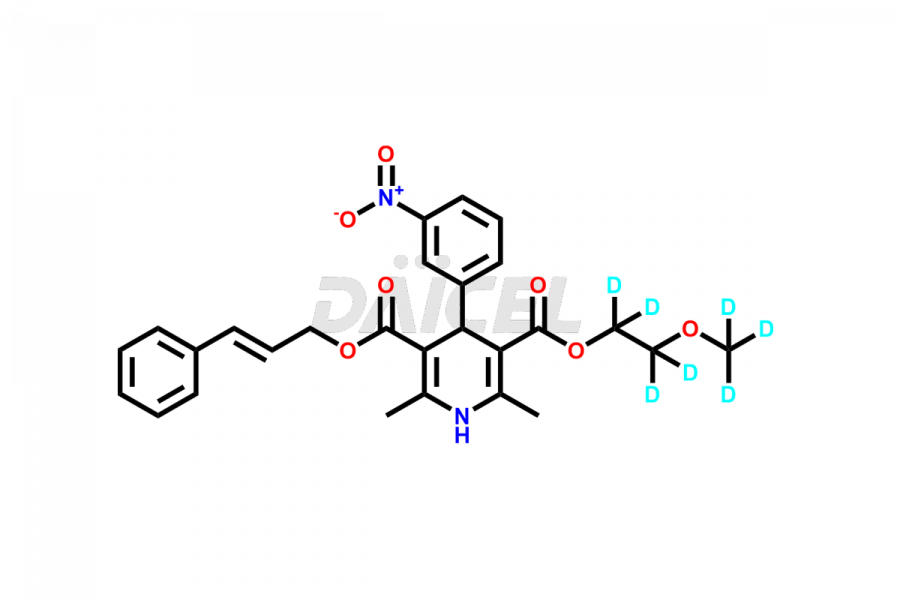
Cilnidipine
General Information
Cilnidipine Impurities and Cilnidipine
Daicel Pharma is a reliable partner that offers high-quality Cilnidipine impurities and isotope-labeled standards. These impurities are essential for evaluating the quality, stability, and safety of Cilnidipine, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Cilnidipine impurities for global delivery to meet the specific needs of our customers.
Cilnidipine [CAS: 132203-70-4] is a dihydropyridine compound. It is a fourth-generation calcium channel blocker. It was developed by both Ajinomoto and Fuji Viscera Pharmaceutical Company of Japan. As a calcium channel blocker (CCB), it relaxes blood vessels and lowers blood pressure. It is an antihypertensive agent and prevents heart attacks.
Cilnidipine: Use and Commercial Availability
Cilnidipine treats hypertension and decreases blood pressure in patients. It also treats cardiovascular diseases due to its vasodilator properties. It prevents angina or stroke in patients by lowering the blood pressure. Cilnidipine is available under various brands as oral formulations of different strengths. The brands are Cinalong, Siscard, Atelec, Nexovas, etc.
Cilnidipine Structure and Mechanism of Action
The chemical name of Cilnidipine is (E)-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)- 3,5-Pyridinedicarboxylic acid 2-methoxyethyl 3-phenyl-2-propenyl ester. The chemical formula for Cilnidipine is C27H28N2O7, and its molecular weight is approximately 492.52 g/mol.
Cilnidipine blocks the incoming calcium and suppresses blood vessel contraction. It acts on the L-type calcium channels of the blood vessels and reduces blood pressure. In addition, Cilnidipine acts at the end of the sympathetic nerve on the N-type calcium channel. It inhibits the norepinephrine emission and suppresses the rise in stress blood pressure.
Cilnidipine Impurities and Synthesis
When synthesizing Cilnidipine, the formation of impurities may affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Cilnidipine. Cilnidipine impurities need control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cilnidipine impurities and labeled standards. A CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity1,2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Cilnidipine impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled standard, Cilnidipine D7. Accompanying the delivery will be a complete characterization report.
References
References:
- Zhang, Xianhua; Zhai, Suodi; Zhao, Rongsheng; Ouyang, Jin; Li, Xiaoguang; Baeyens, Willy R. G., Determination of cilnidipine, a new calcium antagonist, in human plasma using high performance liquid chromatography with tandem mass spectrometric detection, Analytica Chimica Acta, Volume: 600, Issue: 1-2, Pages: 142-146, 2007 DOI: (1016/j.aca.2006.11.072)
- Lee, Heon-Woo; Seo, Ji-Hyung; Lee, Hyun-Su; Jeong, Seo-Young; Cho, Young-Wuk; Lee, Kyung-Tae, Development of a liquid chromatography/negative-ion electrospray tandem mass spectrometry assay for the determination of cilnidipine in human plasma and its application to a bioequivalence study, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 862, Issue: 1-2, Pages: 246-251, 2008 DOI: (10.1016/j.jchromb.2007.11.016)
Frequently Asked Questions
2. What is the main Cilnidipine light degradation impurity?
The Z-isomer of Cilnidipine is the main Cilnidipine light degradation impurity.
3. Which analytical method identifies the main Cilnidipine light degradation impurity?
Liquid chromatography/Q-Orbitrap mass spectrometry (LC/Q-Orbitrap MS) is the analytical method that identifies the main Cilnidipine light degradation impurity.
4. What is the source of nitrosamine impurities in Cilnidipine?
Starting materials, reagents, and solvents containing nitrites or amines can form nitrosamine impurities in Cilnidipine.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.