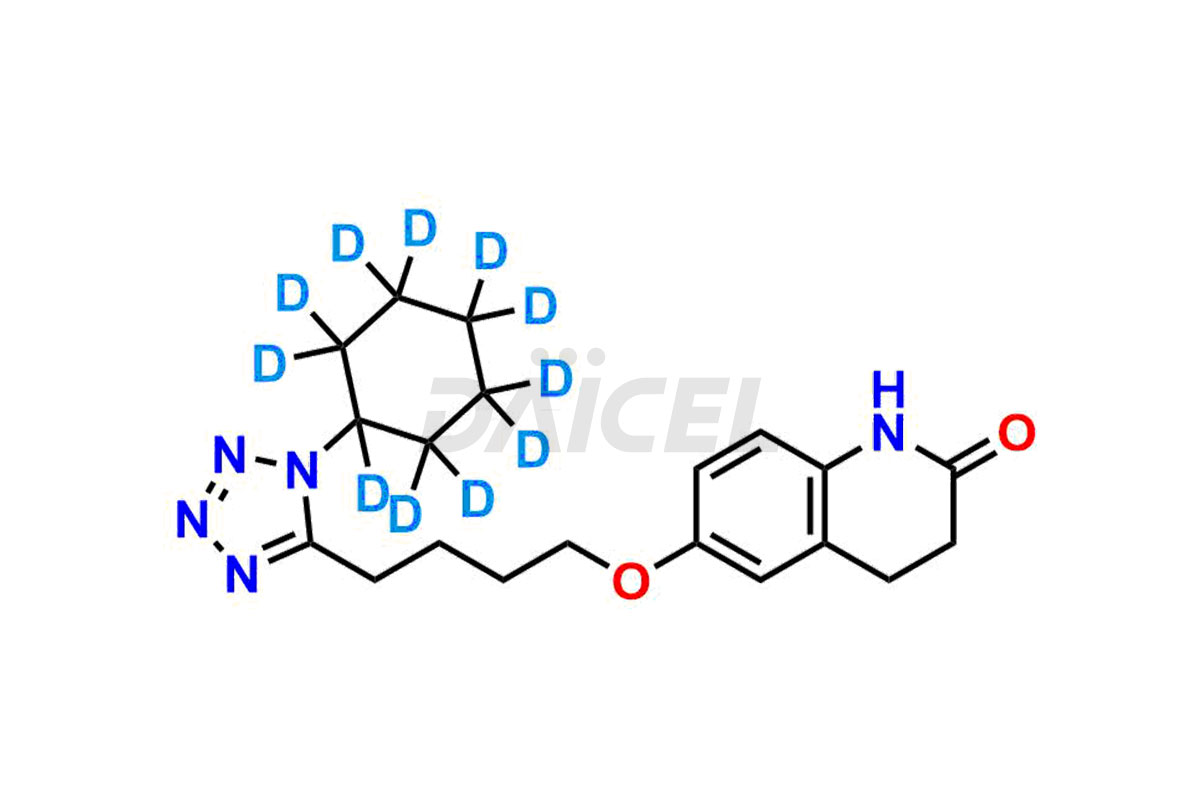
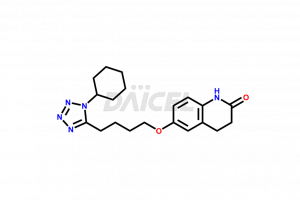
Cilostazol
General Information
Cilostazol Impurities and Cilostazol
Daicel Pharma synthesizes and offers superior-quality Cilostazol impurities and isotope-labeled standards. It is vital for evaluating Cilostazol quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Cilostazol impurities and ensures worldwide delivery.
Cilostazol [CAS: 73963-72-1] is a quinolone derivative. It treats intermittent claudication caused by the narrowing of arteries that supply oxygenated blood in the legs. It improves walking distances in patients with intermittent claudication. Further, it is a Phosphodiesterase III (PDE3) inhibitor. It prevents smooth muscle contraction.
Cilostazol: Use and Commercial Availability
Cilostazol treats intermittent claudication in patients with early-stage peripheral vascular disease. It is an antithrombotic drug that treats chronic peripheral arterial occlusive disease. As an antiplatelet agent, it reduces fatal or non-fatal cardiovascular diseases. It prevents cerebral infarction in patients. Cilostazol is available as an oral tablet under Pletal.
Cilostazol Structure and Mechanism of Action
The chemical name of Cilostazol is 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1H)-quinolinone. The chemical formula for Cilostazol is C20H27N5O2, and its molecular weight is approximately 369.46 g/mol.
Cilostazol inhibits Phosphodiesterase III (PDE3) enzymes that hydrolyze cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP). It increases cAMP in blood vessels and platelets, preventing platelet aggregation and vasodilation.
Cilostazol Impurities and Synthesis
When synthesizing Cilostazol 1, the formation of impurities may affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Cilostazol. Cilostazol impurities need control and monitoring to improve the drug safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cilostazol impurities and labeled standards. A CoA is from a cGMP-compliant analytical facility. It has the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Cilostazol impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable deuterium-labeled standards of Cilostazol Labelled Standard. Accompanying the delivery is a complete characterization report.
References
- Takao Nishi, Kazuyuki Nakagawa, Tetrazolylalkoxycarbostyril derivatives and pharmaceutical compositions containing them, US4277479A, Aug 29, 1979, Otsuka Pharmaceutical Co., Ltd., Japan (https://patents.google.com/patent/US4277479A/en)
- Tata, Prasad N. V.; Fu, Chau-Hwei J.; Browder, Norma J.; Chow, Paul C.; Bramer, Steven L., The quantitative determination of cilostazol and its four metabolites in human liver microsomal incubation mixtures by high-performance liquid chromatography, Journal of Pharmaceutical and Biomedical Analysis, Volume: 18, Issue: 3, Pages: 441-451, 1998 DOI: (10.1016/s0731-7085(98)00052-1)
Frequently Asked Questions
2. Why is it critical to understand the impurity profile of Cilostazol?
It is critical to understand the impurity profile of Cilostazol in order to manufacture safe drugs and to get market approvals from the regulatory authorities.
3. What is the potential genotoxic impurity found in Cilostazol?
5-(4-Chlorobutyl)-1-cyclohexyl-1H-tetrazole is the potential genotoxic impurity found in Cilostazol.
4. Which analytical method can determine the sodium azide content, a genotoxic impurity in Cilostazol?
The High-performance ion chromatographic (HPIC) method can determine the sodium azide content in Cilostazol.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.