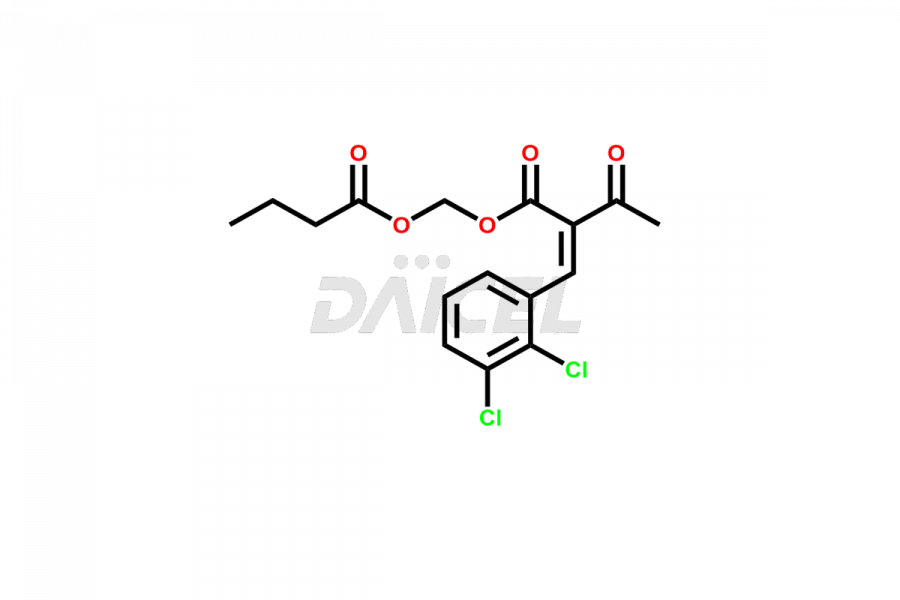
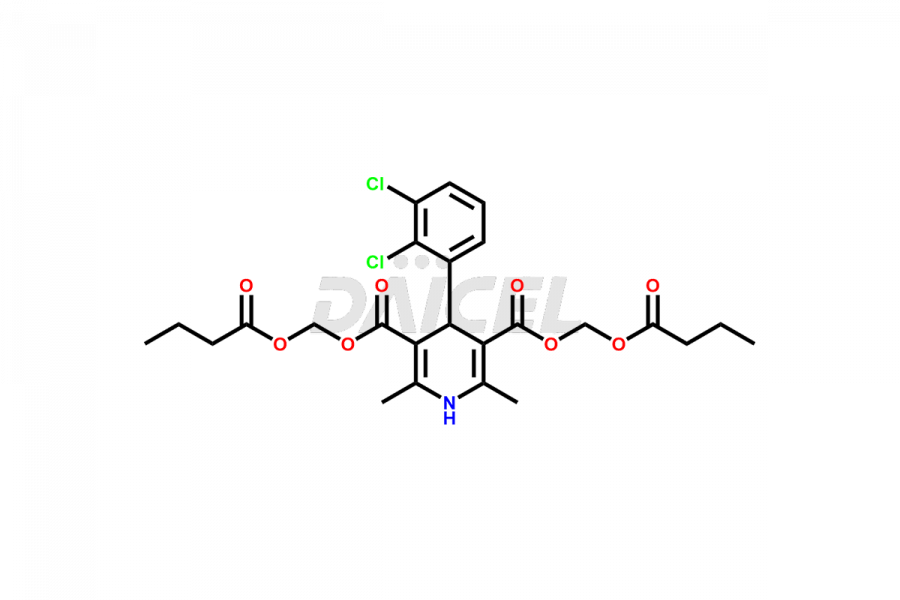
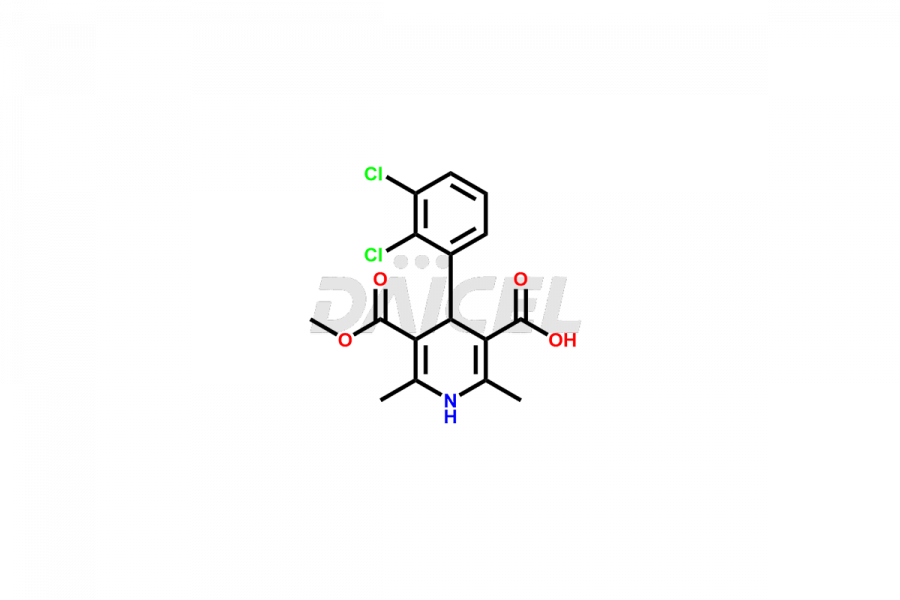
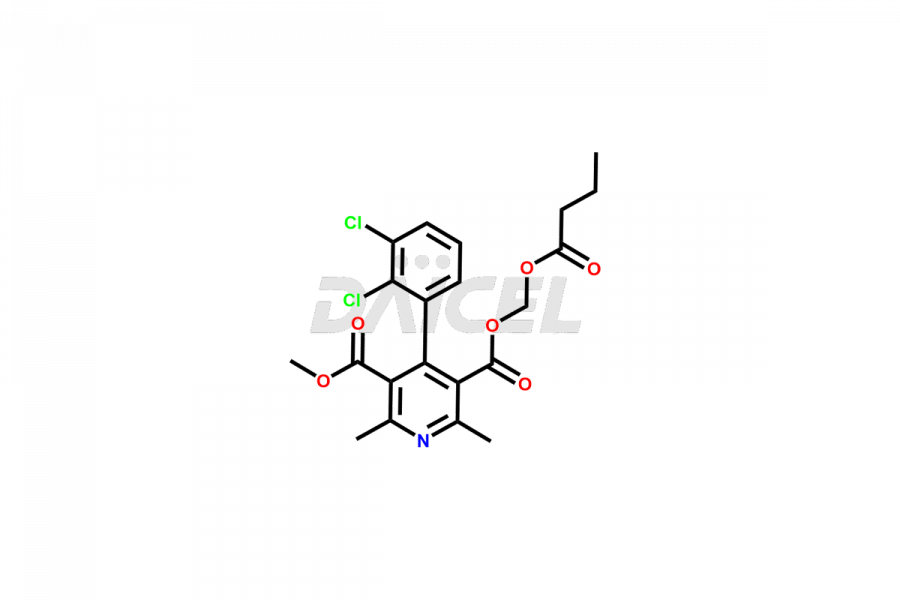
Clevidipine
General Information
Clevidipine Impurities and Clevidipine
Daicel Pharma offers excellent-quality Clevidipine impurities, such as Clevidipine butyrate Impurity CLE 04, Clevidipine Mono Acid impurity, Clevidipine Pyridine impurity, and Clevidipine Dibutyryl Impurity. They are vital for evaluating the quality, stability, and biological safety of Clevidipine. Furthermore, Daicel Pharma specializes in the custom synthesis of Clevidipine impurities and ensures their worldwide delivery.
Clevidipine [CAS: 167221-71-8] is a dihydropyridine calcium channel blocker. It is a third-generation antihypertensive that acts for a short time. It inhibits the L-type calcium channels and causes vasodilation in arterial smooth muscles. It improves oxygen delivery to the myocardial tissue.
Clevidipine: Use and Commercial Availability
Clevidipine reduces blood pressure when oral therapy is not desirable or feasible. It is an arteriolar vasodilator, which is short-acting. Clevidipine is available as a liquid emulsion for intravenous use under Cleviprex. It is effective in treating cardiac surgery adult patients with acute pre-operative and post-operative hypertension.
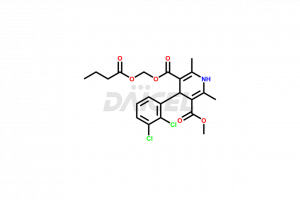
Clevidipine Structure and Mechanism of Action
The chemical name of Clevidipine is 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-Pyridinedicarboxylic acid 3-methyl 5-[(1-oxobutoxy)methyl] ester. The chemical formula for Clevidipine is C21H23Cl2NO6, and its molecular weight is approximately 456.32 g/mol.
Clevidipine relaxes arterial smooth muscle cells and widens arterial lumen, thus lowering blood pressure.
Clevidipine Impurities and Synthesis
During Clevidipine synthesis1, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Clevidipine. And so, Clevidipine impurities must be controlled and monitored throughout the drug’s development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Clevidipine impurities, which includes Clevidipine butyrate Impurity CLE 04, Clevidipine Mono Acid impurity, Clevidipine Pyridine impurity, and Clevidipine Dibutyryl Impurity. The CoA is from a cGMP-compliant analytical facility. It provides complete characterization data2 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Clevidipine impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Clevidipine for bioanalytical research and BA/BE studies. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
FAQ's
References
- Andersson, Kjell Hjalmar; Nordlander, Margareta; Westerlund, Rolf Christer, Short-acting dihydropyridines, WO9512578A1, May 11, 1995, Astra AB, Sweden (https://patents.google.com/patent/WO1995012578A1/en)
- Fakt, Christina; Stenhoff, Helene, Determination of an ultrashort-acting antihypertensive dihydropyridine, clevidipine, in blood using capillary gas chromatography-mass spectrometry and of the primary metabolite using liquid chromatography and fluorescence detection, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 723, Issue: 1 + 2, Pages: 211-219, 1999 DOI: (10.1016/s0378-4347(98)00495-2)
Frequently Asked Questions
2. What is the analytical method to analyze Clevidipine impurities and degradation products?
RP-HPLC method helps analyze and determine Clevidipine impurities and degradation products.
3. How do the degradation products of Clevidipine form?
Acidic, alkaline, and oxidizing conditions result in the formation of the degradation products of Clevidipine.
4. Why is it necessary to remove the degradation products and process-related impurities of Clevidipine from the drug substance?
The presence of the degradation products and process-related impurities of Clevidipine in the drug substance can affect the drug's safety and efficacy. So, it is necessary to remove them from the drug substance.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.