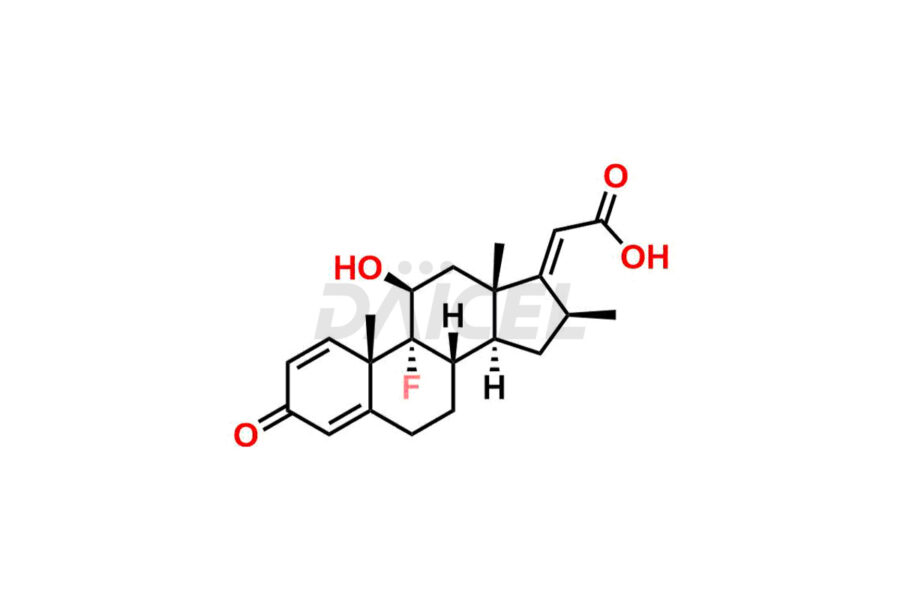
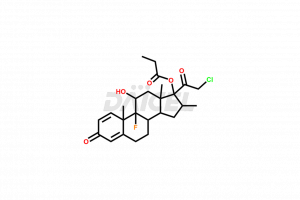
Clobetasolpropionate
General Information
Clobetasol propionate Impurities and Clobetasol propionate
Daicel Pharma offers superior-quality Clobetasol propionate impurities, such as Clobetasol Dipropionate 9-Fluoro Impurity. It is vital for evaluating the quality, stability, and biological safety of Clobetasol propionate l. In addition, Daicel Pharma specializes in the custom synthesis of Clobetasol propionate impurities and ensures their worldwide delivery.
Clobetasol propionate [CAS: 25122-46-7] is a synthetic corticosteroid that treats various skin diseases. It has anti-inflammatory, antimitotic, and immunosuppressive properties and inhibits cytokine production. It reduces swelling, itching, and pain caused by allergic reactions. Clobetasol propionate is in topical formulations.
Clobetasol propionate: Use and Commercial Availability
Clobetasol propionate treats skin diseases such as psoriasis, acute dermatitis, and vulvar lichen sclerosus. It also treats vitiligo, a skin disorder. It is used alone or in combination with azathioprine to treat chronic hand eczema patients. It is marketed under several formulations, such as creams, ointments, gels, and foams. Clobetasol propionate is available under brands such as Clobex, Cormax, Embeline, Impeklo, Impoyz, Olux, Temovate, etc.
Clobetasol propionate Structure and Mechanism of Action
The chemical name of Clobetasol propionate is (11β,16β)-21-Chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)pregna-1,4-diene-3,20-dione. The chemical formula for Clobetasol propionate is C25H32ClFO5, and its molecular weight is approximately 466.97 g/mol.
Clobetasol propionate causes the induction of lipocortins, which are phospholipase A2 inhibitory proteins. It inhibits the release of Arachidonic acid, the precursor of prostaglandins and leukotrienes, inflammatory mediators.
Clobetasol propionate Impurities and Synthesis
When synthesizing Clobetasol propionate 1, the formation of impurities may affect drug safety and efficacy. They form during the synthetic process, storage, or purification of Clobetasol propionate. Clobetasol propionate impurities need control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Clobetasol propionate impurities, which includes Clobetasol Dipropionate 9-Fluoro Impurity. We provide CoA from a cGMP-compliant analytical facility, which consists of the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Clobetasol propionate impurity or degradation product. In addition, Daicel Pharma offers highly purified stable isotope-labeled standards of Clobetasol propionate for bioanalytical research and BA/BE studies. Daicel Pharma provides a complete characterization report accompanying the delivery.
References
- Elks, Joseph; Phillipps, Gordon Hanley, 9 alpha,21-dihalopregnane compounds, GB1253831A, Nov 17, 1971, Glaxo Laboratories Ltd. (https://www.lens.org/lens/search/patent/list?q=GB1253831)
- Gagliardi, L.; De Orsi, D.; Manna, F.; Tonelli, D., HPLC determination of clobetasol propionate in cosmetic products, Journal of Liquid Chromatography & Related Technologies, Volume: 23, Issue: 3, Pages: 355-362, 2000 DOI: (10.1081/jlc-100101456)
Frequently Asked Questions
2. How do we identify Clobetasol propionate degradation products?
LC-MS/MS analysis and H NMR analysis help identify Clobetasol propionate degradation products.
3. Which analytical method analyzes Clobetasol propionate impurities in the drug substance?
RP-HPLC method analyzes Clobetasol propionate impurities in the drug substance.
4. Why is it necessary to remove Clobetasol propionate impurities?
Clobetasol propionate impurities can affect drug efficacy and pose health risks. So, it needs removal from Clobetasol propionate.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.