LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma synthesizes Clomiphene impurities of exceptional quality, such as 4-Hydroxy Clomiphene (Mixture of Z and E Isomers), Clomiphene N-Oxide, N-Desethyl Clomiphene Hydrochloride (Mixture of Z and E Isomers), and ZuClomiphene Citrate salt. These impurities are crucial to assess the purity, reliability, and safety of an active pharmaceutical ingredient, Clomiphene. Besides, Daicel Pharma provides custom synthesis of Clomiphene impurities to meet clients’ demands for delivery worldwide.
Clomiphene [CAS: 911-45-5] is a triphenylethylene nonsteroidal ovulatory stimulant that exhibits estrogenic and anti-estrogenic activities. It helps in promoting ovulation and treats infertility in women.
Clomiphene, also known as Clomiphene citrate, treats infertility in women. Clomiphene treats anovulatory or oligo-ovulatory infertility to induce ovulation in patients. It helps patients with conditions such as polycystic ovarian syndrome (PCOS), post-oral-contraceptive amenorrhea, etc. Popular brand names include Clomid, Clomiphene Citrate, Milophene, and Serophene.
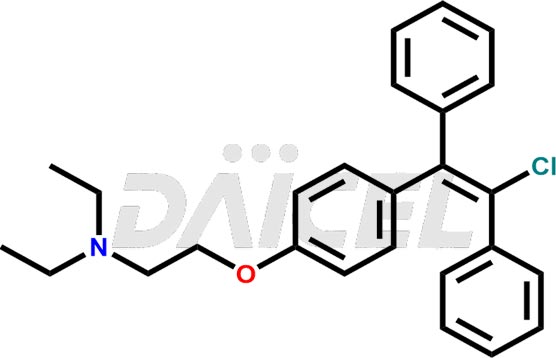
The chemical name of Clomiphene is 2-[4-(2-Chloro-1,2-diphenylethenyl)phenoxy]-N, N-diethylethanamine. Its chemical formula is C26H28ClNO, and its molecular weight is approximately 406.0 g/mol.
Clomiphene competes with estrogen for estrogen-receptor-binding sites. It interacts with estrogen-receptor-containing tissues like the pituitary, hypothalamus, endometrium, ovary, cervix, and vagina.
Impurities in Clomiphene develop during the synthetic process, storage, or due to degradation. They can potentially impact the drug’s safety, efficacy, and quality. The analysis and control of Clomiphene impurities are essential to ensure product quality and patient safety. Common Clomiphene impurities include related substances, degradation products, and residual solvents. Analytical methods such as HPLC and LC help analyze and quantify these impurities, allowing their control within acceptable limits during synthesis.
Daicel Pharma offers a Certificate of Analysis (CoA) for Clomiphene impurity standards, such as 4-Hydroxy Clomiphene (Mixture of Z and E Isomers), Clomiphene N-Oxide, N-Desethyl Clomiphene Hydrochloride (Mixture of Z and E Isomers), and ZuClomiphene Citrate salt, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity1. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Clomiphene impurities or degradation products and labeled compounds to assess the effectiveness of generic Clomiphene. We also offer EnClomiphene D10 Citrate salt and ZuClomiphene D10 Citrate salt, deuterium-labeled Clomiphene compounds useful in bio-analytical research, such as BA/BE studies. A complete characterization report accompanies every delivery.
Clomiphene impurities are monitored in the final drug product through routine quality control testing, where samples are analyzed to ensure compliance with regulatory requirements regarding impurity content.
Some Clomiphene impurities can harm patients due to reduced drug efficacy, increased toxicity, or unexpected side effects. Therefore, controlling them is crucial for patient safety.
Methanol or Acetonitrile are the solvents used for analyzing many impurities in Clomiphene.
Clomiphene impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA). Highly sensitive Clomiphene impurities such as 4-HydroxyClomiphene (Mixture of Z and E Isomers) are stored in an inert atmosphere.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.