Cortisone
General Information
Cortisone Impurities and Cortisone
Daicel Pharma offers exceptional quality Cortisone impurities, such as 21-Dehydro Cortisone Impurity (mixture of the aldehyde & the hydrated form). They are vital for evaluating Cortisone quality, stability, and biological safety. Further, Daicel Pharma specializes in the custom synthesis of Cortisone impurities and ensures worldwide delivery.
As a synthetic corticosteroid, Cortisone [CAS: 53-06-5] treats inflammation. It mimics the action of cortisol, produced by the adrenal gland of the human body. It is a C-21 steroid, which converts to its active metabolite, hydrocortisone.
Cortisone: Use and Commercial Availability
Cortisone is an anti-inflammatory agent and an immunosuppressant. It treats inflammatory disorders such as rheumatoid arthritis and ankylosing arthritis. It is effective in treating patients with active ulcerative colitis. It helps in the management of autoimmune diseases. Cortisone is available in oral, injectable, and topical formulations. Many companies sell Cortisone under brands such as Cortone, Cortisate, Cortogen, etc.
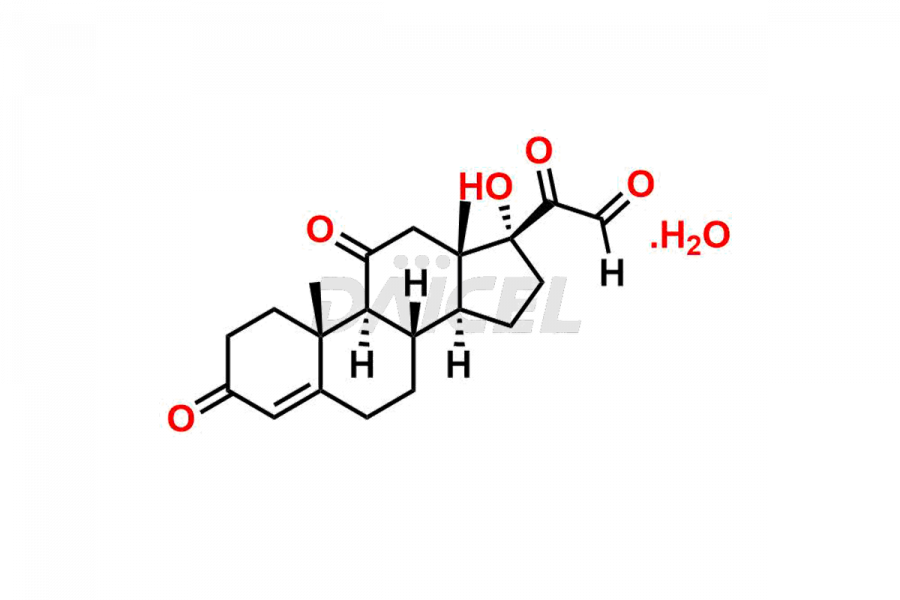
Cortisone Structure and Mechanism of Action
The chemical name of Cortisone is 17,21-Dihydroxypregn-4-ene-3,11,20-trione. The chemical formula for Cortisone is C21H28O5, and its molecular weight is approximately 360.44 g/mol.
Cortisone suppresses the migration of polymorphonuclear neutrophils and fibroblasts. It reduces capillary permeability and modifies transcription and synthetic rate of inflammatory mediators.
Cortisone Impurities and Synthesis
During Cortisone synthesis, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Cortisone. And so, Cortisone impurities must be controlled and monitored throughout the drug’s development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cortisone impurities, which includes 21-Dehydro Cortisone Impurity (mixture of the aldehyde & the hydrated form). The CoA is from a cGMP-compliant analytical facility. It provides complete characterization data1 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Cortisone impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Cortisone for bioanalytical research and BA/BE studies. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
FAQ's
References
- Newsome, H. H. Jr.; Clements, A. S.; Borum, E. H., Simultaneous assay of cortisol, corticosterone, 11-deoxycortisol, and cortisone in human plasma, Journal of Clinical Endocrinology and Metabolism, Volume: 34, Issue: 3, Pages: 473-83, 1972 DOI: (1210/jcem-34-3-473)
- Olson, Michael C., Analysis of adrenocortical steroids in pharmaceutical preparations by high-pressure liquid-liquid chromatography, Journal of Pharmaceutical Sciences, Volume: 62, Issue: 12, Pages: 2001-7, 1973 DOI: (10.1002/jps.2600621223)
- Williams, Patricia A.; Biehl, Edward R., High-pressure liquid chromatographic determination of corticosteroids in topical pharmaceuticals, Journal of Pharmaceutical Sciences, Volume: 70, Issue: 5, Pages: 530-4, 1981 DOI: (1002/jps.2600700517)
Frequently Asked Questions
2. Which analytical method identifies and analyzes Cortisone degradation products?
HPLC method helps identify and analyze Cortisone degradation products.
3. Why is it necessary to remove the Cortisone degradation products from the drug substance?
Cortisone degradation products in the drug substance can affect the drug's safety, quality, and efficacy. So, it is necessary to remove them from the drug substance.
4. How do we identify unknown Cortisone impurities?
LC-MS/MS helps identify unknown Cortisone impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.