Cromolyn
General Information
Cromolyn Impurities and Cromolyn
Daicel Pharma is a reliable source in synthesizing excellent-quality Cromolyn impurities, such as Cromolyn Tricarboxylic acid analog. They are vital for checking Cromolyn’s quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Cromolyn impurities and ensures their global delivery.
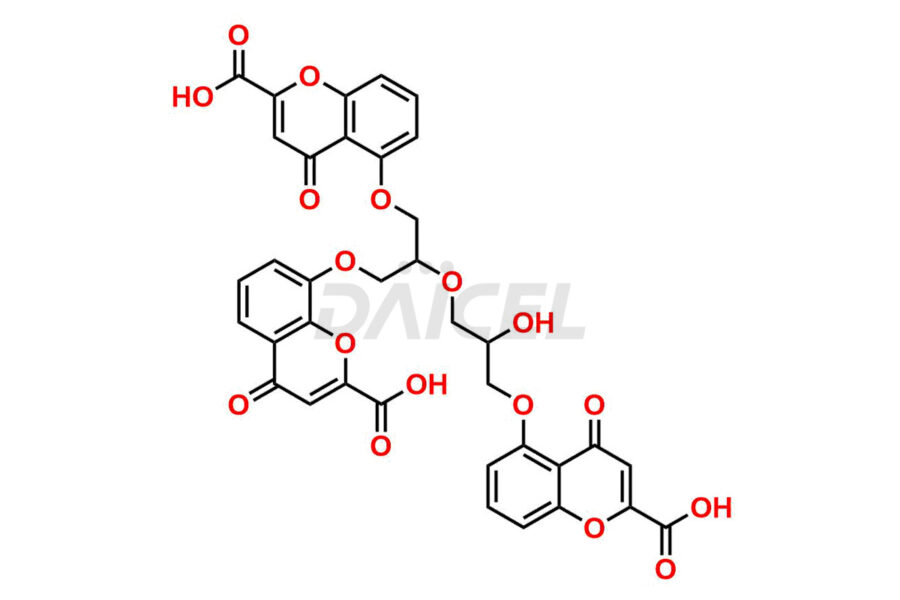
Cromolyn [CAS: 16110-51-3] is a bis-chromone derivative of glycerol. Also known as Cromoglicate, it treats asthma, both allergic and exercise-induced. It prevents the release of inflammatory mediators, histamines, and leukotrienes. In addition, it treats conjunctivitis, allergic rhinitis, and ulcerative colitis.
Cromolyn: Use and Commercial Availability
Cromolyn treats allergic rhinitis, bronchial asthma, and other allergic eye conditions like keratitis and keratoconjunctivitis. Further, it treats systemic mastocytosis in patients. Cromolyn also prevents and relieves nasal allergies, hay fever symptoms, etc. Drug manufacturers sell Cromolyn in various formulations such as metered spray, oral, ophthalmic solutions, etc. Cromolyn is available under Crolom, Cromoptic, Gastrocom, Intal, Nasalcrom, Opticrom, etc.
Cromolyn Structure and Mechanism of Action
The chemical name of Cromolyn is 5,5′-[(2-Hydroxy-1,3-propanediyl)bis(oxy)]bis[4-oxo-4H-1-benzopyran-2-carboxylic acid]. The chemical formula for Cromolyn is C23H16O11, and its molecular weight is approximately 468.37 g/mol.
Cromolyn decreases intracellular calcium accumulation. It reduces pulmonary mast cell degranulation. Further, it prevents histamine release and inflammatory leukotrienes.
Cromolyn Impurities and Synthesis
During Cromolyn synthesis1, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Cromolyn. Controlling and monitoring of Cromolyn impurities is necessary throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cromolyn impurities, which includes Cromolyn Tricarboxylic acid analog. Daicel Pharma offers CoA from a cGMP-compliant analytical facility. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Cromolyn impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable isotope-labeled Cromolyn standard. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
- Colin Fitzmaurice, Thomas Brian Lee and Roger Edward Collingwood Altounyan, Substituted bis-(2-carboxy-chromonyl-oxy) derivatives and preparation and pharmaceutical compositions thereof, GB1144905A, Mar 12, 1969, Fisons Pharmaceuticals Ltd., United Kingdom (https://www.lens.org/lens/search/patent/list?q=GB1144905)
- Gardner, John J., Determination of sodium cromoglycate in human urine by high-performance liquid chromatography on an anion-exchange column, Journal of Chromatography, Biomedical Applications, Volume: 305, Issue: 1, Pages: 228-32, 1984, DOI: (10.1016/s0378-4347(00)83335-6)
Frequently Asked Questions
2. How do we detect the unknown Cromolyn impurities and degradation products in the drug product?
Liquid chromatography-mass spectroscopy can detect the unknown impurities and degradation products of Cromolyn in the drug product.
3. Which analytical method determines the presence of Cromolyn impurities in the drug substance?
A stability-indicating isocratic HPLC method determines the presence of Cromolyn impurities in the drug substance.
4. Why is it necessary to eliminate Cromolyn impurities from the drug product?
Cromolyn impurities can affect drug safety, efficacy, and quality. So, it is necessary to remove Cromolyn impurities from the drug product.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.