Cyclizine
General Information
Cyclizine Impurities and Cyclizine
Daicel Pharma is a reliable source in synthesizing excellent-quality Cyclizine impurities and labeled standards. They are vital for evaluating the quality, stability, and biological safety of Cyclizine. Furthermore, Daicel Pharma specializes in the custom synthesis of Cyclizine impurities and ensures their global delivery.
Cyclizine [CAS: 82-92-8] is an N-alkylpiperazine derivative and antiemetic drug. It blocks the histamine actions and is a histamine H1 antagonist. It treats nausea and vomiting associated with motion sickness, vertigo, and other vestibular disorders. It also demonstrates anticholinergic action.
Cyclizine: Use and Commercial Availability
Cyclizine is available under brands such as Valoid, Marzine, Emoquil, etc. Many manufacturers market it as oral or parenteral formulations. Cyclizine prevents and relieves symptoms of nausea and vomiting due to motion sickness. Further, it is a part of the cancer treatment therapy. Cyclizine also treats sickness caused by Meniere’s disease and vertigo.
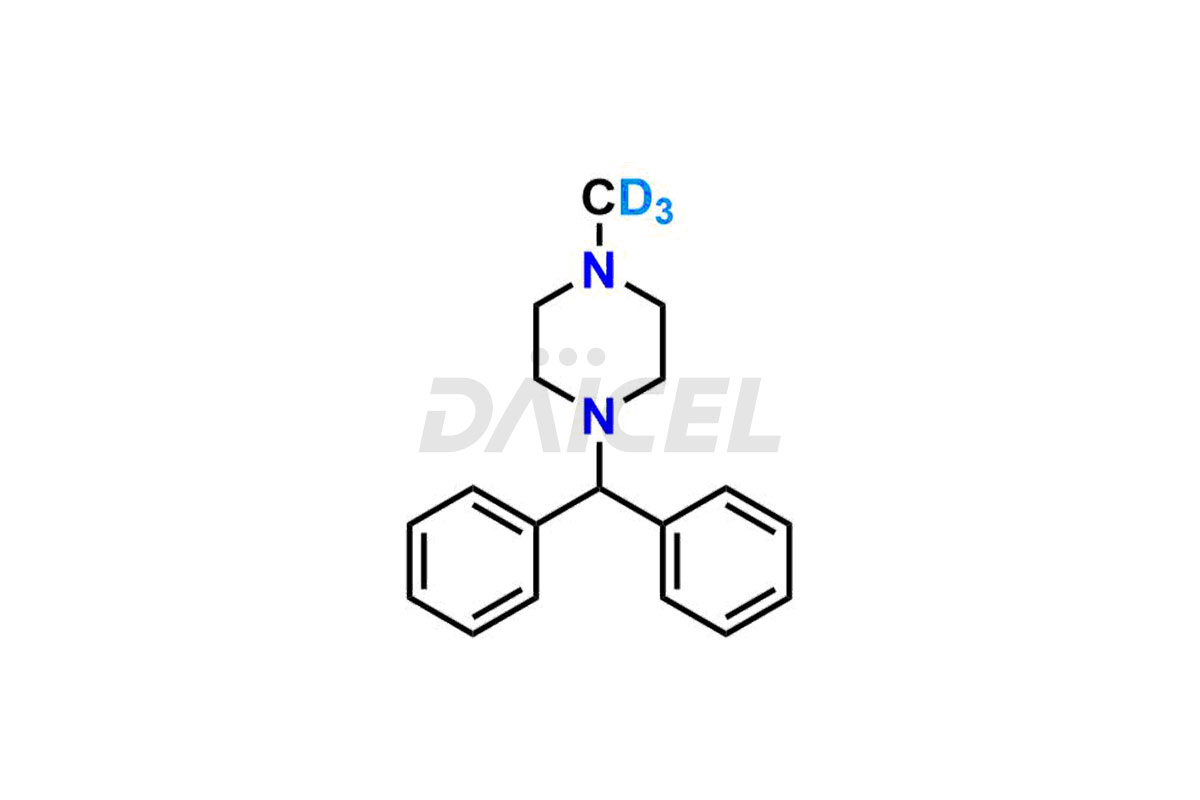
Cyclizine Structure and Mechanism of Action
The chemical name of Cyclizine is 1-(Diphenylmethyl)-4-methylpiperazine. The chemical formula for Cyclizine is C18H22N2, and its molecular weight is approximately 266.38 g/mol.
Cyclizine inhibits the histamine receptors in the brain’s vomiting center and decreases activity along the pathways.
Cyclizine Impurities and Synthesis
Impurities form during Cyclizine synthesis1 that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Cyclizine. These impurities must be controlled and monitored throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cyclizine impurities and labeled standards. Daicel Pharma offers CoA from a cGMP-compliant analytical facility. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Cyclizine impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable deuterium-labeled Cyclizine Labelled Standard. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
- Improvements in and relating to the preparation of piperazine compounds, GB655839A, Aug 1, 1951, Burroughs Wellcome & Co. (U.S.A.) Inc., Wellcome Foundation Ltd. (https://www.lens.org/lens/search/patent/list?q=GB655839)
- Land, G.; Dean, K.; Bye, A., Determination of cyclizine and norcyclizine in plasma and urine using gas-liquid chromatography with nitrogen selective detection, Journal of Chromatography, Biomedical Applications, Volume: 222, Issue: 1, Pages: 135-40, 1981 DOI: (10.1016/s0378-4347(00)81043-9)
Frequently Asked Questions
2. How do we detect the Cyclizine impurities in the drug substance?
UPLC can detect the Cyclizine impurities in the drug substance.
3. Which analytical method detects the presence of Cyclizine degradation products in the drug product?
RP-HPLC method is the analytical method that determines the presence of Cyclizine degradation products in the drug product.
4. How do the Cyclizine degradation products form?
Cyclizine degradation products form under acid, alkaline, oxidative, heat, and light conditions.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.