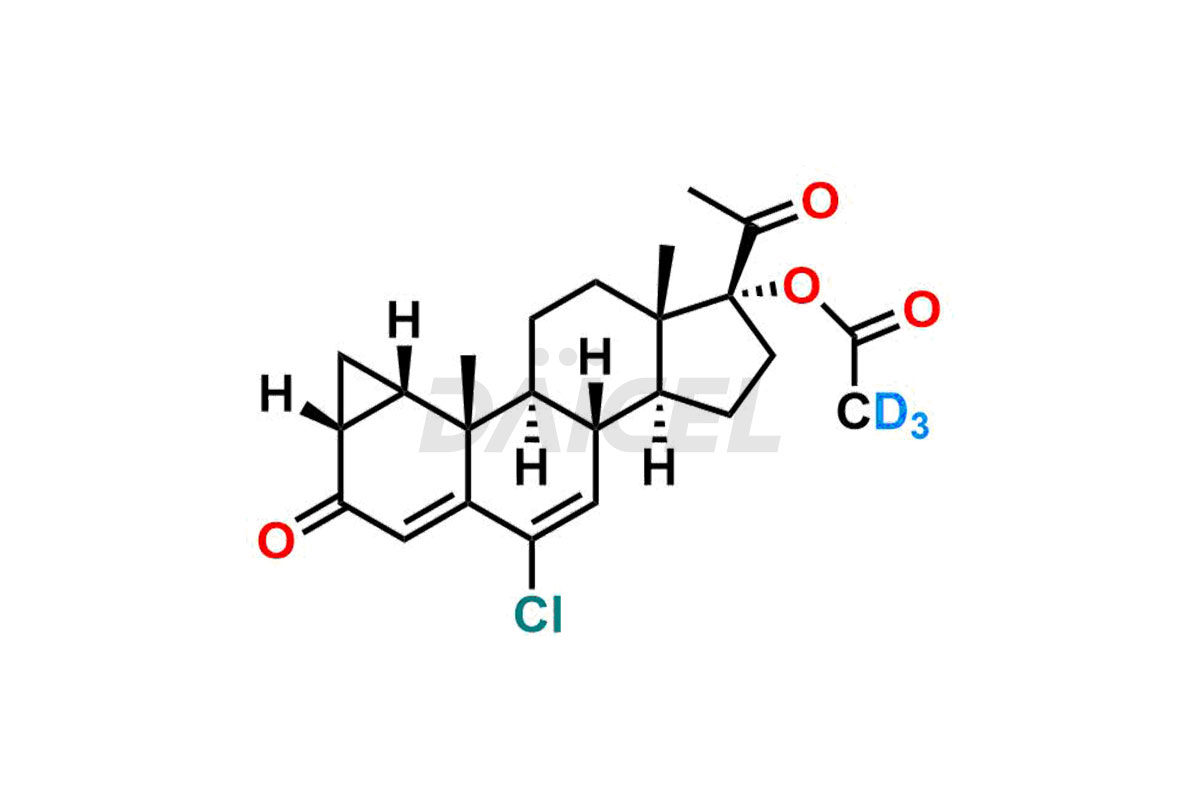
Cyproterone Acetate
General Information
Cyproterone acetate Impurities and Cyproterone acetate
Daicel Pharma is a reliable source in synthesizing excellent-quality Cyproterone acetate impurities and labeled standards. They are vital for evaluating the quality, stability, and biological safety of Cyproterone acetate. Furthermore, Daicel Pharma specializes in the custom synthesis of Cyproterone acetate impurities and ensures their global delivery.
Cyproterone acetate [CAS: 427-51-0] is a steroid ester, which is an antiandrogenic drug. It is a synthetic progesterone derivative. It suppresses androgens with progestogenic activity. Cyproterone acetate stops the growth of testosterone-sensitive tumor cells. It also acts as a contraceptive agent. It decreases the activity of 5α-reductase in the skin.
Cyproterone acetate: Use and Commercial Availability
Cyproterone acetate treats patients with prostatic cancer. It is for contraceptive activity in males. It prevents the actions of androgens. Further, Cyproterone acetate with ethinyl estradiol treats women with severe acne and hirsutism. It is an oral formulation available under Cyproterone, Androcur, Cyprostat, etc.
Cyproterone acetate Structure and Mechanism of Action
The chemical name of Cyproterone acetate is (1β,2β)-17-(Acetyloxy)-6-chloro-1,2-dihydro-3′H-cyclopropa[1,2]pregna-1,4,6-triene-3,20-dione. The chemical formula for Cyproterone acetate is C24H29ClO4, and its molecular weight is approximately 416.94 g/mol.
Cyproterone acetate blocks the dihydrotestosterone binding to the specific receptors in the prostatic carcinoma cell. It inhibits the secretion of luteinizing hormone and causes lowered production of testicular testosterone.
Cyproterone acetate Impurities and Synthesis
During Cyproterone acetate synthesis1, impurities form that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Cyproterone acetate. Cyproterone acetate impurities must be controlled and monitored throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cyproterone acetate impurities and labeled standards. Daicel Pharma offers CoA from a cGMP-compliant analytical facility. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Cyproterone acetate impurity or degradation product. In addition, Daicel Pharma offers highly purified, stable deuterium-labeled standards, Cyproterone Acetate-13C2D3, and Cyproterone Acetate-D3. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
FAQ's
References
- Rudolf Wiechert, Berlin-Lichterfelde, 6-chloro-1, 2alpha-methylene-delta6-17alpha-hydroxyprogesterone compounds and compositions, US3234093A, Apr 29, 1961, Schering A.-G. (https://www.lens.org/lens/search/patent/list?q=US3234093)
- Cannell, G. R.; Mortimer, R. H.; Thomas, M. J., High-performance liquid chromatographic estimation of cyproterone acetate in human plasma, Journal of Chromatography, Biomedical Applications, Volume: 226, Issue: 2, Pages: 492-7, 1981 DOI: (0.1016/s0378-4347(00)86087-9)
Frequently Asked Questions
2. What are the conditions responsible for the degradation of Cyproterone acetate?
Cyproterone acetate degradation occurs under acidic, alkaline, and peroxide conditions.
3. How do Cyproterone acetate impurities arise in the drug substance?
During drug manufacturing, process-related impurities form in the drug substance due to unreacted raw materials, catalysts, reagents, and solvents.
4. What is the reason for removing Cyproterone acetate impurities from the drug?
Cyproterone acetate impurities need to be removed from the drug so that the drug is safe, stable, and effective for human use.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.