Cysteine
General Information
Cysteine Impurities and Cysteine
Daicel Pharma is a reliable source in synthesizing excellent-quality Cysteine impurities, such as Ac D Cysteine. They are vital for evaluating Cysteine quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Cysteine impurities and ensures their global delivery.
Synthesized from methionine, Cysteine [CAS: 3374-22-9] ] is an amino acid. It is essential to meet the nutritional requirements of infants less than a month old. It gets synthesized in the liver of the human body. It is a necessary source of sulfur in the human body. It also acts as a reducing agent and helps in bread making. L-cysteine [CAS: 52-90-4] is its active form.
Cysteine: Use and Commercial Availability
Cysteine is an essential amino acid that meets the nutritional requirements of neonates. It is also vital for senior citizens and individuals with metabolic diseases. It has antioxidant properties. Cysteine treats individuals with severe liver disease. It prevents liver and kidney damage due to acetaminophen overdose. Cysteine is available under Nouress and Elcys as injectables.
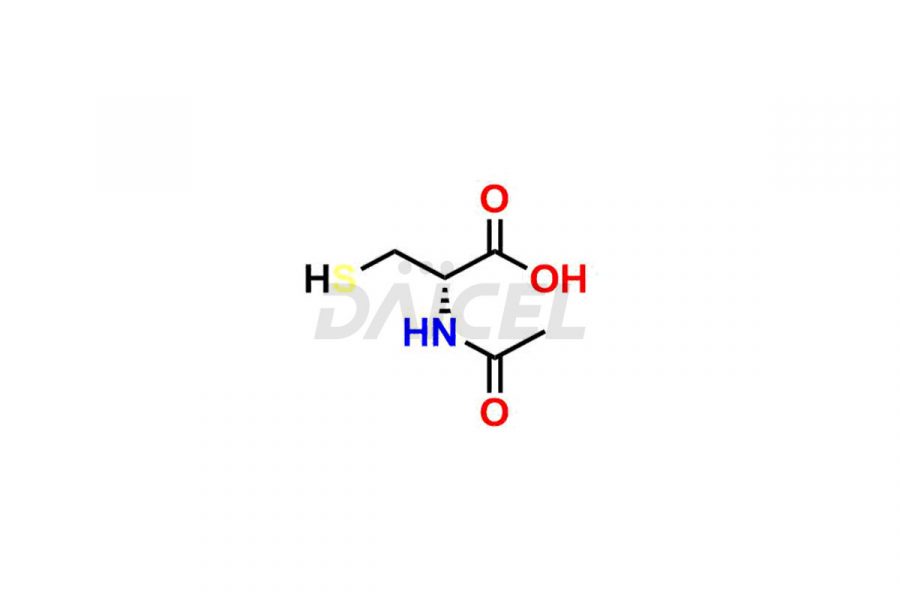
Cysteine Structure and Mechanism of Action
The chemical name of Cysteine is 2-Amino-3-sulfanylpropanoic acid. The chemical formula for Cysteine is C3H7NO2S, and its molecular weight is approximately 121.16 g/mol.
Cysteine acts as a precursor substrate for taurine and glutathione. It is essential for the functioning of the human immune system.
Cysteine Impurities and Synthesis
Impurities form during Cysteine synthesis1 that may affect the safety and efficacy of the drug. They form during the synthetic process, purification, or storage of Cysteine. Cysteine impurities must be controlled and monitored throughout the drug development process.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Cysteine impurities, which includes Ac D Cysteine. Daicel Pharma offers CoA from a cGMP-compliant analytical facility. It provides complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional analytical data on request. Daicel Pharma can prepare any unidentified Cysteine impurity or degradation product. In addition, Daicel Pharma offers a highly purified, stable isotope-labeled standard of Cysteine. The clients of Daicel Pharma can expect a complete characterization report on delivery.
References
- Process for the preparation of Racemic cysteine and inactive cystine, as well as DL-S-benzyl-N-acetylcysteine, GB1252580A, Nov 10, 1971, Diamalt A.-G. (https://www.lens.org/lens/search/patent/list?q=GB1252580)
- Olieman, C., Comments on cystine and cysteine analysis as the same phenylthiocarbamyl derivatives by HPLC, Chromatographia, Volume: 35, Issue: 5-6, Pages: 344, 1993 DOI: (10.1007/bf02277522)
Frequently Asked Questions
2. Which analytical method identifies the presence of Cysteine impurities in the drug substance?
HPLC method is the analytical method that identifies the presence of Cysteine impurities in the drug substance.
3. Why is it necessary to eliminate nitrosamine impurities from Cysteine?
Nitrosamine impurities are carcinogenic and affect drug safety. So, it is necessary to remove nitrosamine impurities from Cysteine.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.