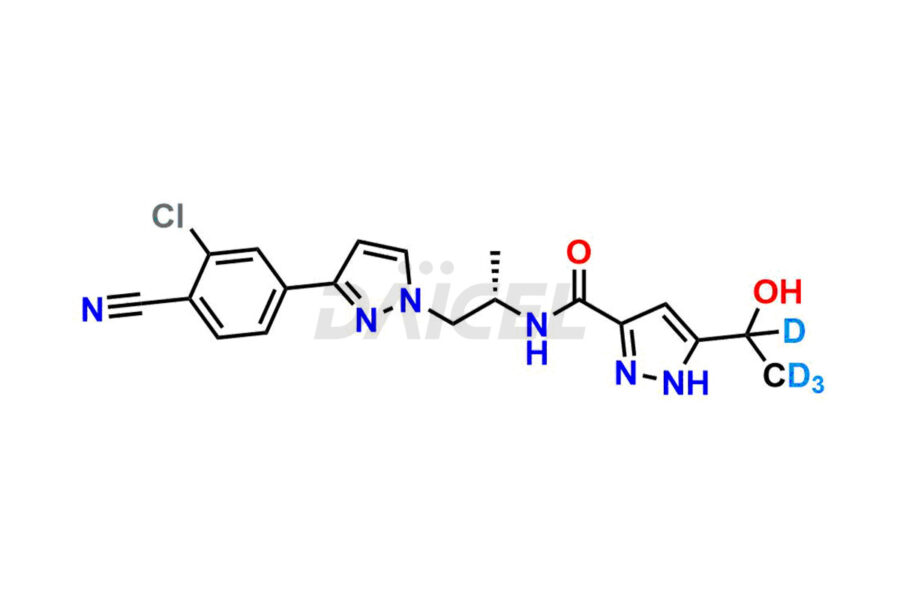
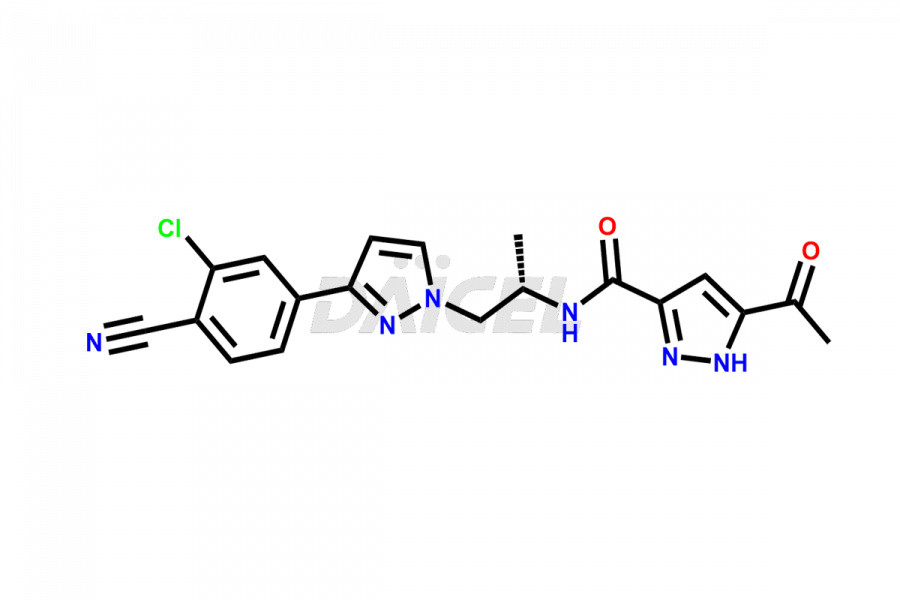
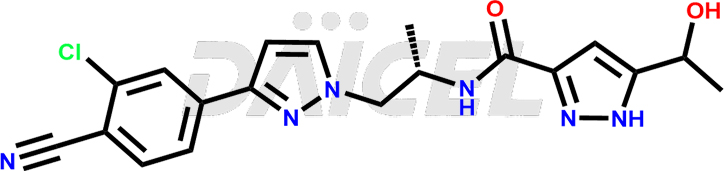
Darolutamide
General Information
Darolutamide Impurities and Darolutamide
Daicel Pharma synthesizes high-quality Darolutamide impurity, KetoDarolutamide, which is crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Darolutamide. Moreover, Daicel Pharma offers custom synthesis of Darolutamide impurities and delivers them globally.
Darolutamide [CAS: 1297538-32-9] is a medicine that functions as an androgen receptor antagonist and has potential anti-cancer properties. It treats non-metastatic prostate cancer that has become resistant to hormone therapy.
Darolutamide: Use and Commercial Availability
Nubeqa is a brand name for Darolutamide for treating adult males with non-metastatic castration-resistant prostate cancer (nmCRPC) at risk of metastasis. It binds to the receptor (target) of androgens, such as testosterone, and blocks them from stimulating prostate cancer cells from growing.
Darolutamide Structure and Mechanism of Action 
The chemical name of Darolutamide is N-[(1S)-2-[3-(3-Chloro-4-cyanophenyl)-1H-pyrazol-1-yl]-1-methylethyl]-5-(1-hydroxyethyl)-1H-pyrazole-3-carboxamide. Its chemical formula is C19H19ClN6O2, and its molecular weight is approximately 398.8 g/mol.
Darolutamide inhibits androgen binding, androgen receptor nuclear translocation, and Androgen receptor-mediated transcription.
Darolutamide Impurities and Synthesis
During the synthetic process1, Darolutamide impurities form that affect the quality and safety of the drug. These impurities must be monitored and controlled to ensure the drug is safe and effective for patients. It requires a thorough understanding of the potential impurities, their preparation, and appropriate measures to limit them.
Daicel provides a Certificate of Analysis (CoA) for the Darolutamide impurity standard, KetoDarolutamide. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Darolutamide impurity or degradation product and offer labeled compounds to quantify the efficacy of Darolutamide. Daicel offers Darolutamide-D4, a deuterium-labeled Darolutamide standard used in bio-analytical research such as BA/BE studies. We give a complete characterization
References
FAQ's
References
- Wohlfahrt, Gerd; Tormakangas, Olli; Salo, Harri; Hoglung, Lisa; Karjalainen, Arja; Knuuttila, Pia; Holm, Patrick; Rasku, Sirpa; Vesalainen, Anniina, Androgen Receptor Modulating Compounds, Orion Corporation, Finland, EP2493858B1, July 2, 2014
- Nawle, Yogita; Daroi, Pratibha; Sonawane, Bhushan; Bandre, Aditi; Munipalli, Vijaya Kumar; Warde, S. U.; Singh, Raman Mohan, Development, validation of HPTLC method for darolutamide in tablet dosage form, World Journal of Pharmaceutical Research, Volume: 11, Issue: 6Spec.Iss.,Pages: 868-881, 2022
Frequently Asked Questions
How are Darolutamide impurities controlled?
Impurities in Darolutamide are controlled by using appropriate manufacturing processes, quality control procedures, and analytical methods to detect and quantify impurities.
What is the impact of impurities on the pharmacokinetics of Darolutamide?
Impurities can affect the pharmacokinetics of Darolutamide, as they can alter the drug's absorption, distribution, metabolism, and excretion in the body.
Which solvent helps in the analysis of Darolutamide impurities?
Methanol is a solvent used in analyzing many impurities in Darolutamide.
What are the temperature conditions required to store Darolutamide impurities?
Darolutamide impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.