LOAD MORE
You're viewed 9 of 23 products
Daicel Pharma synthesizes Dasatinib impurities of exceptional quality, such as Dasatinib Carboxylic Acid, Dasatinib carboxylic acid ethyl ester, Dasatinib impurity-A, Hydroxymethyl Dasatinib, etc. These impurities are crucial to assess the purity, reliability, and safety of Dasatinib, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Dasatinib impurities to meet clients’ demands for delivery worldwide.
Dasatinib [CAS: 302962-49-8] is an antineoplastic agent that treats chronic myelogenous leukemia (CML) and acute lymphatic leukemia (ALL).
Dasatinib, available under the tradename Sprycel, is a medication for treating different types of leukemia in adult and pediatric patients. It is also effective in treating chronic, accelerated, or blast phase CML when there is resistance or intolerance to prior therapy, including imatinib. Additionally, Dasatinib is used for Ph+ acute lymphoblastic leukemia (ALL) and lymphoid blast CML in cases of resistance or intolerance to prior therapy.
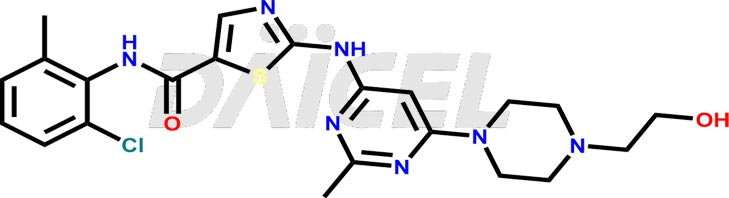
The chemical name of Dasatinib is N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazole carboxamide. Its chemical formula is C22H26ClN7O2S, and its molecular weight is approximately 488.0 g/mol.
Dasatinib inhibits multiple tyrosine kinases like BCR-ABL, EPHA2, SRC, etc. It inhibits chronic myeloid leukemia (CML) growth cell lines overexpressing BCR-ABL.
Impurities in Dasatinib develop through various pathways, including degradation of the active ingredient, interaction with excipients or impurities in raw materials, and oxidative processes. They can impact the quality, efficacy, and safety of Dasatinib. Analytical methods such as liquid chromatography and mass spectrometry help analyze and identify Dasatinib impurities. The control measures include specifying impurity level limits, implementing good manufacturing practices, and conducting testing, throughout the manufacturing process1. Strict quality control and monitoring ensure that Dasatinib batches comply with regulatory requirements and maintain high purity standards, ultimately ensuring the efficacy and safety of the drug.
Daicel Pharma offers a Certificate of Analysis (CoA) for Dasatinib impurity standards, such as Dasatinib Carboxylic Acid, Dasatinib carboxylic acid ethyl ester, Dasatinib impurity-A, Hydroxymethyl Dasatinib, etc., generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Dasatinib impurities or degradation products and labeled compounds to assess the effectiveness of generic Dasatinib. We also offer Dasatinib D4, a deuterium-labeled Dasatinib standard useful in bio-analytical research, such as BA/BE studies. A complete characterization report accompanies every delivery.
Regulatory authorities, such as the United States Pharmacopeia (USP) and the International Conference on Harmonization (ICH), provide guidelines and limits for impurity levels in pharmaceutical products, including Dasatinib.
Impurities in Dasatinib are identified and characterized using analytical techniques such as high-resolution mass spectrometry (HRMS) and comparison with reference standards.
The steps to minimize impurity formation during Dasatinib synthesis are optimizing reaction conditions, controlling temperature and pH, using appropriate catalysts and reagents, and implementing effective purification techniques.
Dasatinib impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.