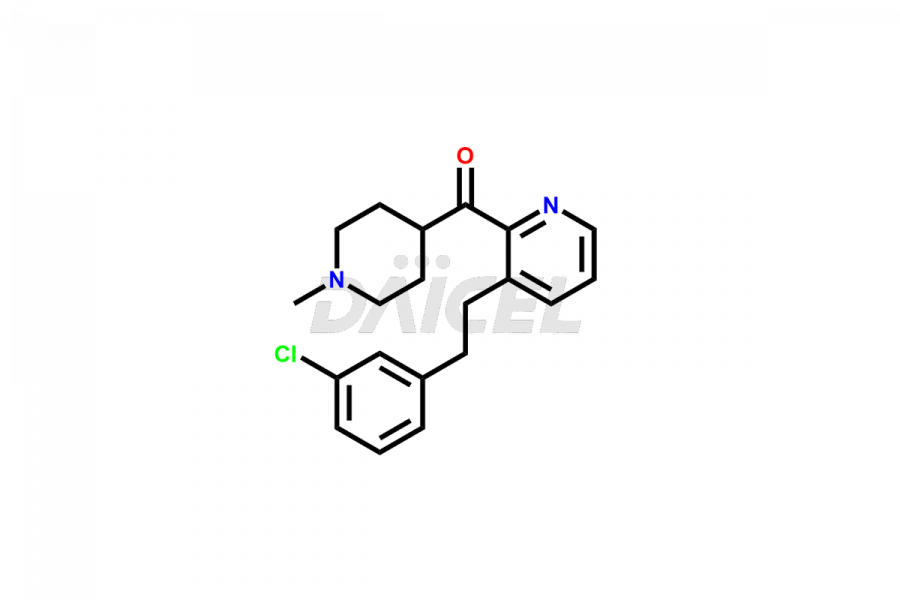
Desloratadine
General Information
Desloratadine Impurities and Desloratadine
Daicel Pharma offers high-quality Desloratadine impurities, Desloratadine Methanone Impurity, and genotoxic NDSRI N-nitroso-desloratadine impurity. They are vital for evaluating Desloratadine quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Desloratadine impurities and ensures their worldwide delivery.
Desloratadine [CAS: 100643-71-8] is a major metabolite of Loratadine. It is an antihistamine to alleviate symptoms associated with allergies such as rhinitis and chronic urticaria. It is a tricyclic H1 inverse agonist.
Desloratadine: Use and Commercial Availability
Desloratadine, a piperidine derivative with prolonged action, exhibits selective H1 antihistaminergic properties. Unlike some antihistamines, Desloratadine does not induce drowsiness as it does not readily penetrate the central nervous system. Its roles include serving as an H1-receptor antagonist, an anti-allergic agent, a cholinergic antagonist, and a drug metabolite. Desloratadine is prescribed to alleviate allergic symptoms. Marketed under the trade name Clarinex, it treats allergies.
Desloratadine Structure and Mechanism of Action


The chemical name of Desloratadine is 8-Chloro-6,11-dihydro-11-(4-piperidinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine. The chemical formula for Desloratadine is C19H19ClN2, and its molecular weight is approximately 310.8g/mol.
Desloratadine is a long-acting tricyclic histamine antagonist. It has selective H1-receptor histamine antagonist activity. It inhibits histamine release from human mast cells in vitro.
Desloratadine Impurities and Synthesis
During the synthesis1 of Desloratadine, meticulous steps can maintain its purity and therapeutic efficacy. Despite these precautions, impurities may occur, affecting the drug’s quality. Advanced purification methods such as chromatography and crystallization can minimize impurities, preserving the integrity of Desloratadine.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Desloratadine impurities, which includes Desloratadine Methanone Impurity and genotoxic NDSRI N-nitroso-desloratadine impurity. The CoA is from a cGMP-compliant analytical facility and encompasses complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we give additional data like 13C-DEPT and CHN. Daicel Pharma also prepares any unidentified Desloratadine impurity or degradation product. Furthermore, Daicel Pharma offers highly purified isotope-labeled standards of Desloratadine for bioanalytical research and BA/BE studies. We provide a complete characterization report upon delivery.
References
FAQ's
References
- Hartmann, Fred; Ilner, Wolfgang R., Quantitative estimation of unconjugated cortisol, cortisone, corticosterone, cortexone, and cortexolone by thin-layer chromatography, Research in Experimental Medicine, Volume: 161, Issue: 2, Pages: 165-74, 1973, DOI: (10.1007/BF01855109)
- Villani, Frank J., 8-chloro-6,l 1-dihydro-l l-(4-piperidylidene)-5h-benzo[5,6]cyclohepta[l,2-b]pyridine and its salts, processes for the production thereof and pharmaceutical compositions containing these compounds,WO8503707A1, Aug 29, 1985, Schering Corp., United States
Frequently Asked Questions
What are the steps if unexpected impurities occur during routine quality control testing of Desloratadine batches?
If unexpected Desloratadine impurities are detected, investigations can identify their sources, assess potential risks to patient safety, and implement corrective actions to prevent recurrence.
Can Desloratadine impurities affect the drug's bioavailability in the body?
Some Desloratadine impurities may alter the bioavailability of Desloratadine, affecting its absorption, distribution, metabolism, and excretion in the body.
What are the safety measures to take during the storage of Desloratadine impurities?
Safety measures during the storage of Desloratadine impurities include proper labeling, secure storage conditions, and regular monitoring for any signs of degradation.
What are the uses of Desloratadine impurities?
Desloratadine impurities help in pharmaceutical research, product development, ANDA and DMF filing, quality control (QC), method validation, and stability studies.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.