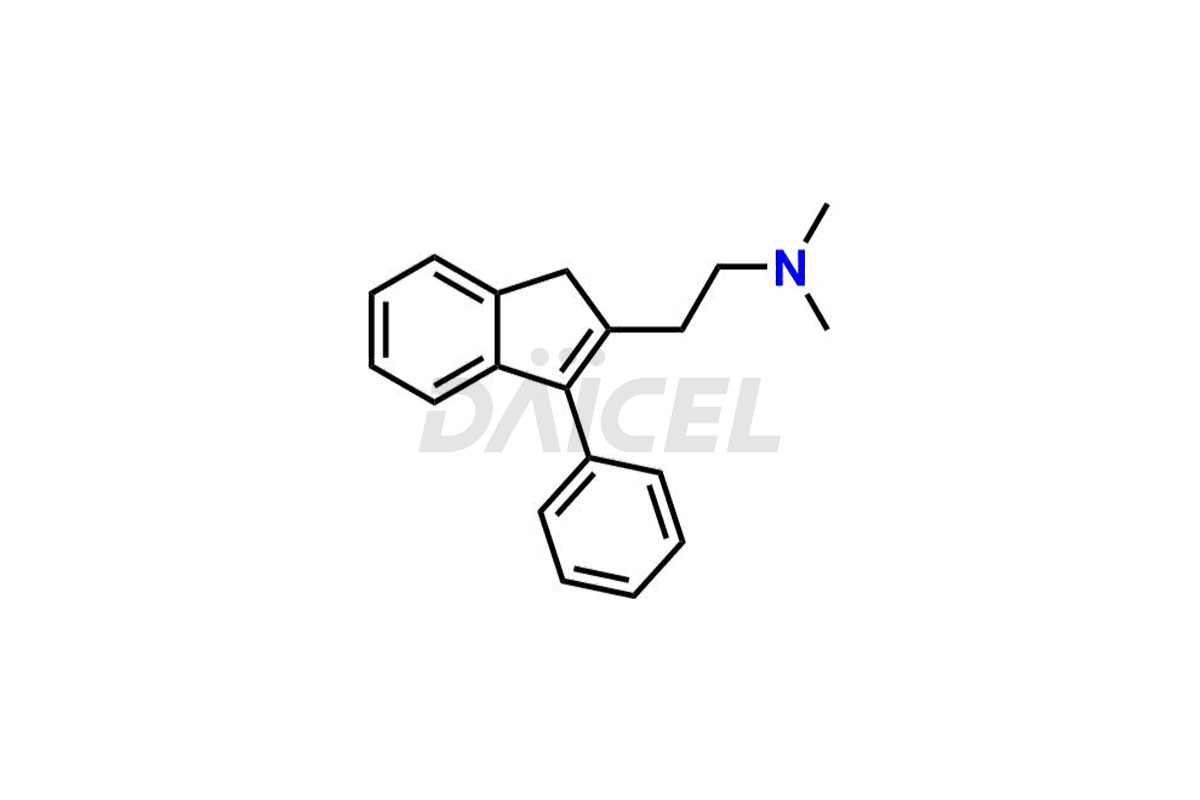
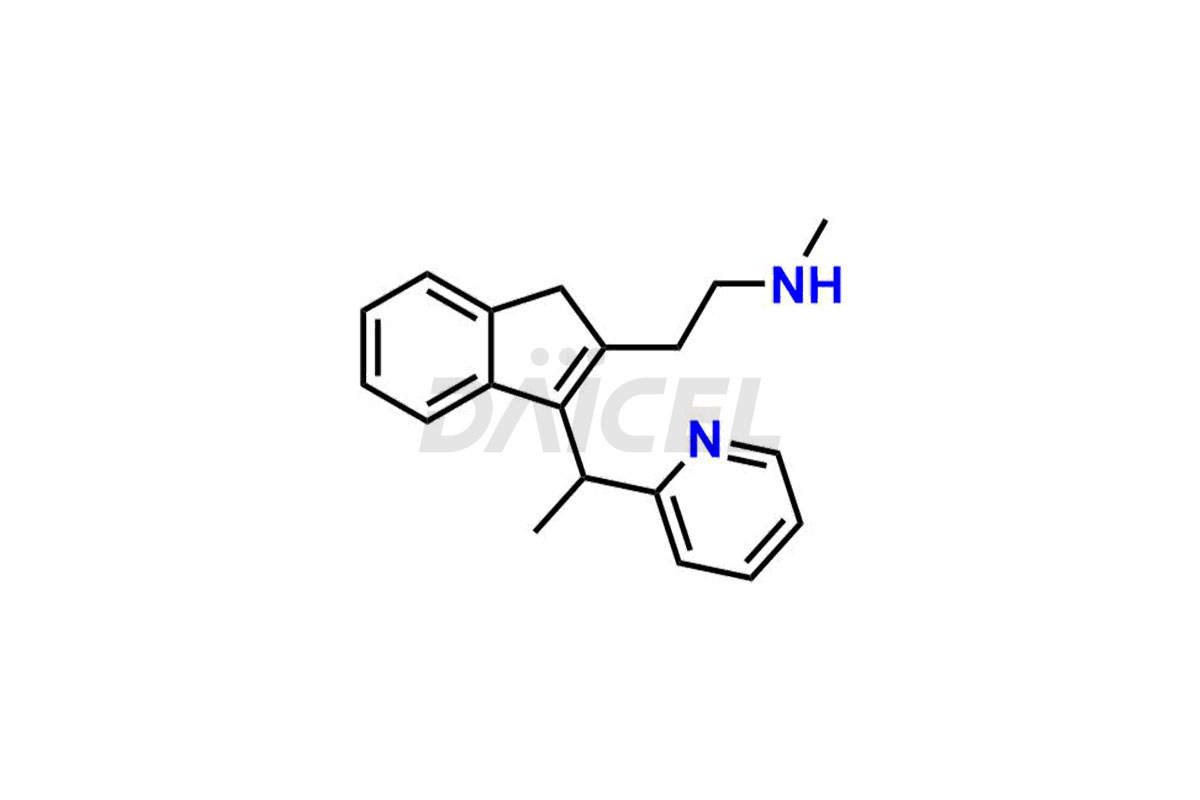
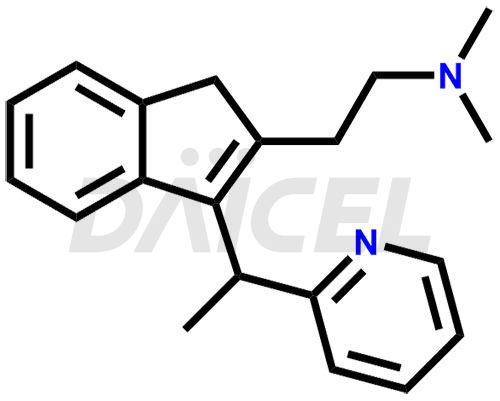
Dimetindene
General Information
Dimetindene Impurities and Dimetindene
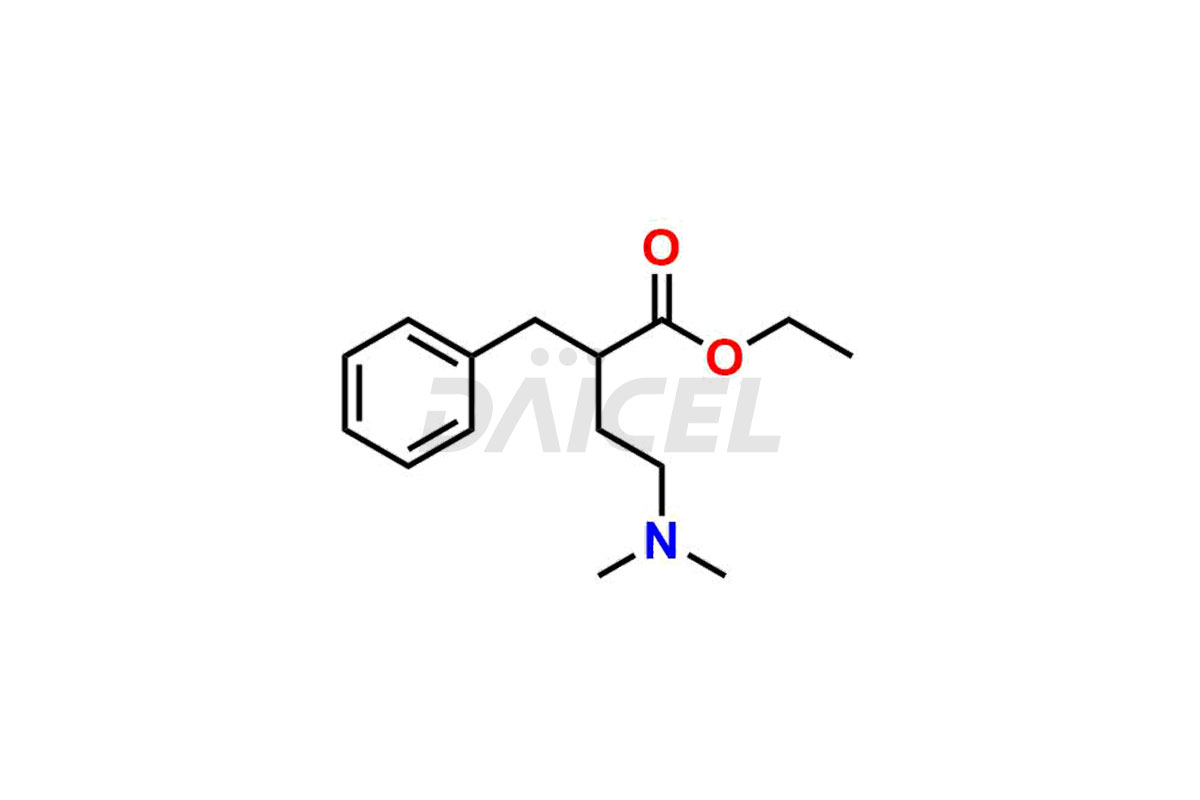
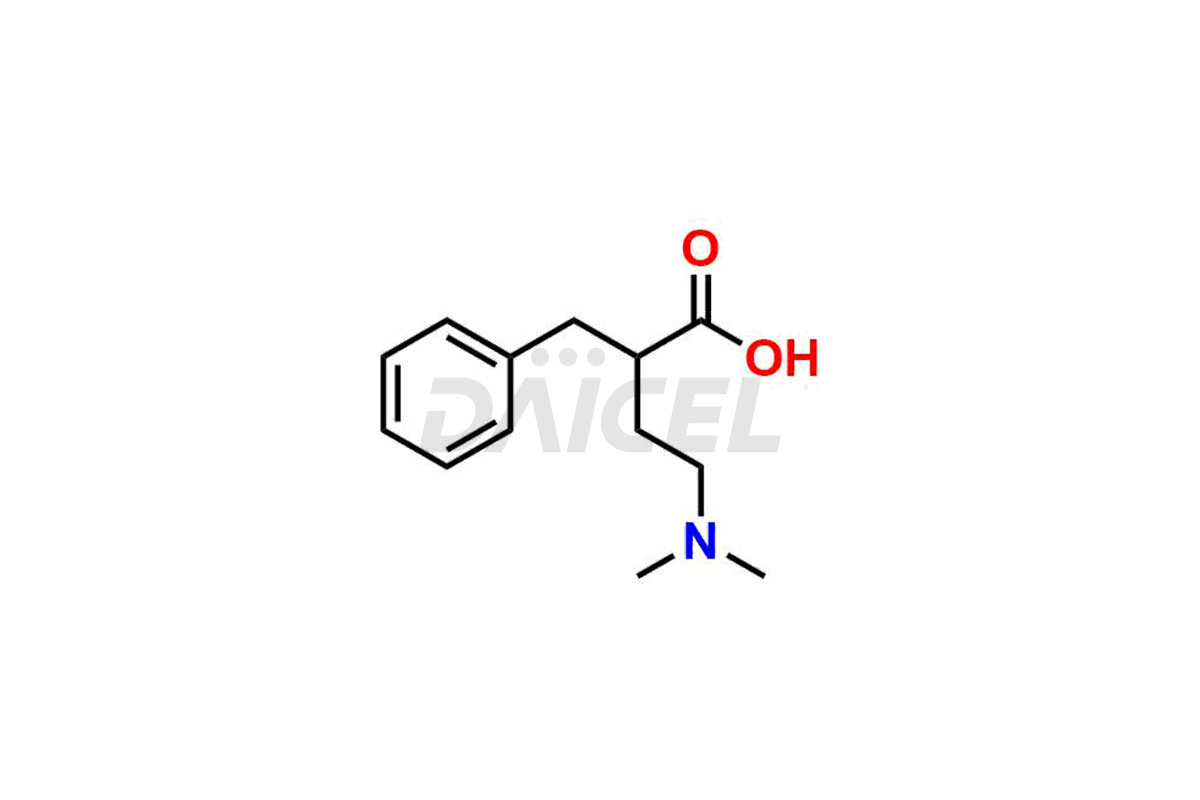
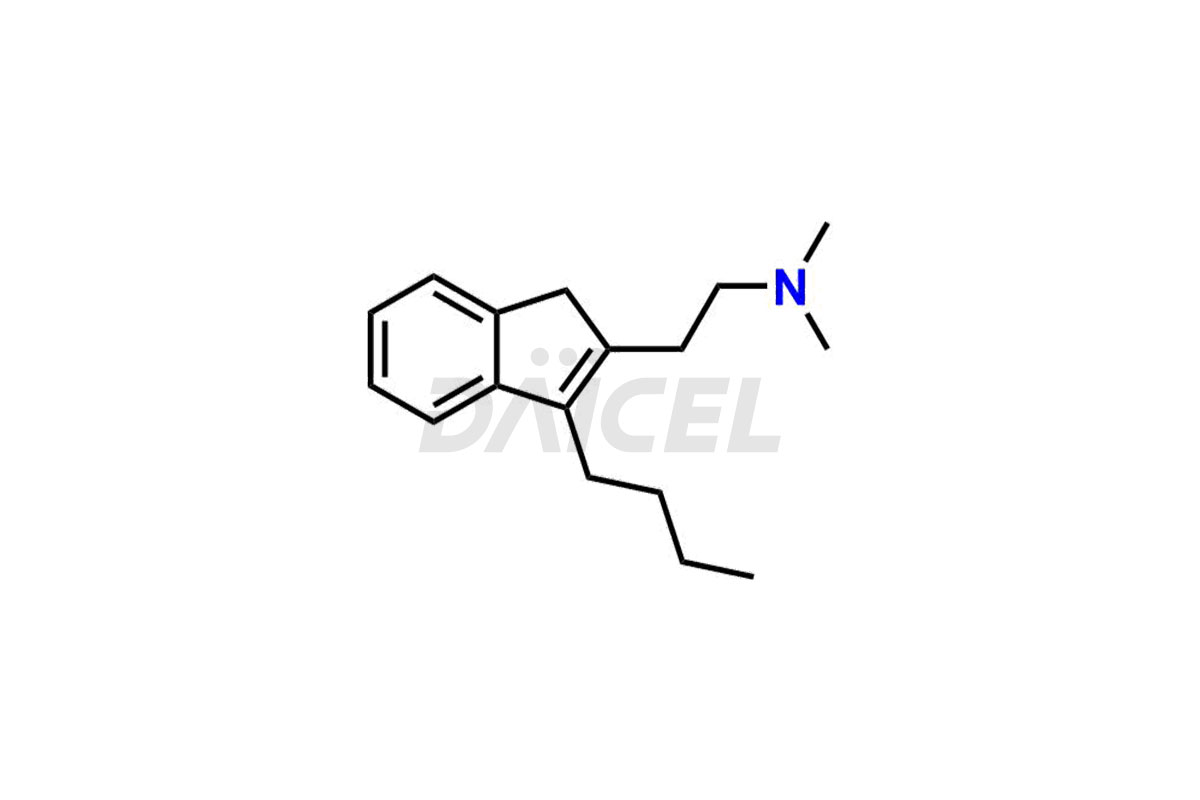
Daicel Pharma specializes in offering high-quality impurities for Dimetindene, a crucial active pharmaceutical ingredient. These impurities, including Dimetindene EP Impurity – E, Dimetindene EP Impurity- A, Dimetindene EP Impurity-C, Dimetindene EP Impurity-D, Dimetindene EP Impurity-F, Dimetindene EP Impurity-G, Dimetindene EP impurity-H, and Dimetindene EP impurity-I, play a vital role in assessing the purity, reliability, and safety of Dimetindene. Daicel Pharma also offers a customized synthesis of Dimetindene impurities to cater to clients’ requirements, with worldwide delivery options available.
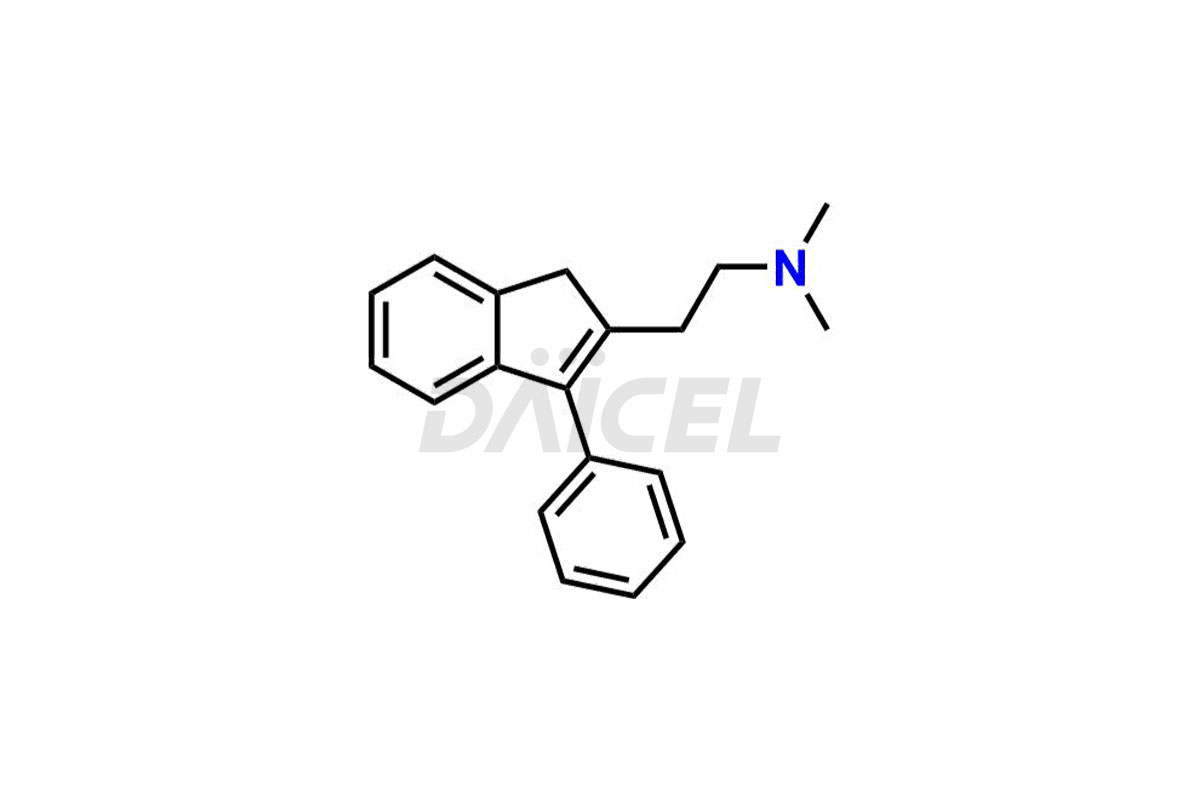
Dimetindene [CAS: 5636-83-9], an indene compound, acts as an antihistamine. It is a first-generation H1 antagonist. It treats allergic reactions such as urticaria, rhinitis, pruritus, and common cold symptoms.
Dimetindene: Use and Commercial Availability
Dimetindene, available under the brand name Fenistil treats various disorders. It treats pruritus in eruptive skin diseases such as chickenpox. Additionally, it is effective in relieving pruritus of different origins, except pruritus caused by cholestasis. Dimetindene treats the upper respiratory tract allergies like hay fever and perennial rhinitis, food and drug allergies, and urticaria. It is an adjuvant in managing eczema and other pruritic allergic dermatoses.
Dimetindene Structure and Mechanism of Action
The chemical name of Dimetindene is N, N-Dimethyl-3-[1-(2-pyridinyl)ethyl]-1H-indene-2-ethanamine. Its chemical formula is C20H24N2, and its molecular weight is approximately 292.4 g/mol.
Dimetindene inhibits the interaction of histamine with histamine receptors and treats allergies.
Dimetindene Impurities and Synthesis
The formation of impurities in Dimetindene can occur during various stages such as synthesis1, storage, or degradation processes. Analyzing and controlling these impurities is crucial to ensure the safety and quality of Dimetindene. Impurity analysis helps identify, quantify, and characterize unwanted substances, thus establishing appropriate specifications and limits. Impurities in Dimetindene can be minimized by effective control, ensuring regulatory compliance, maintaining product stability, and other harmful effects.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Dimetindene impurity standards, including Dimetindene EP Impurity – E, Dimetindene EP Impurity- A, Dimetindene EP Impurity-C, Dimetindene EP Impurity-D, Dimetindene EP Impurity-F, Dimetindene EP Impurity-G, Dimetindene EP impurity-H and Dimetindene EP impurity-I. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Dimetindene impurities or degradation products. Every delivery accompanies a complete characterization report.
References
FAQ's
References
- Huebner, Charles F., Process For Preparation Of 2(N, N-Di-Loweralkyl - Amino - Loweralkyl)-3[(2-Pyridyl)-(R1)methyl]-Indenes, Ciba Pharmaceutical Products, Inc., US2947756A, August 2, 1960
- Wermeille, M. M.; Huber, G. A., Gas-liquid-chromatographic determination of free dimethindene in human serum and urine at low concentrations, Journal of Chromatography, Biomedical Applications, Volume: 228, Pages: 187-94, 1982
Frequently Asked Questions
Are there specific analytical methods used for impurity control in Dimetindene?
Many validated analytical methods, such as high-performance liquid chromatography (HPLC), help detect and quantify impurities in Dimetindene.
What measures help to minimize Dimetindene impurities during the storage of the drug?
The proper storage conditions, such as temperature control and protection from light and moisture, help to minimize impurity formation.
Which solvent helps in the analysis of Dimetindene impurities?
Methanol or Acetonitrile are the solvents used in analyzing many impurities in Dimetindene.
What are the temperature conditions required to store Dimetindene impurities?
Dimetindene impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.