Doxepin
General Information
Doxepin Impurities and Doxepin
Daicel Pharma offers high-quality impurities for Doxepin, an active pharmaceutical ingredient. These impurities, including E-Doxepin and Z-Doxepin, play a vital role in assessing the purity, reliability, and safety of Doxepin. Daicel Pharma also offers a customized synthesis of Doxepin impurities to cater to client requirements, with worldwide delivery options available.
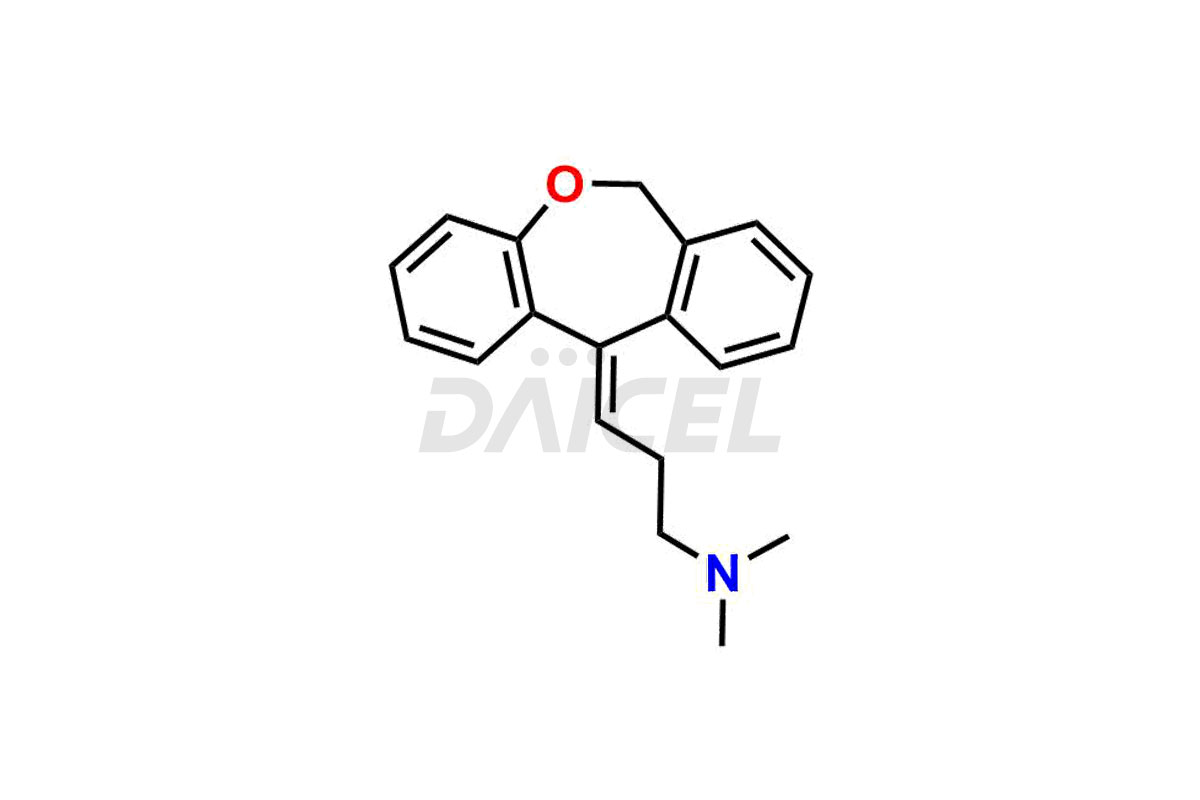
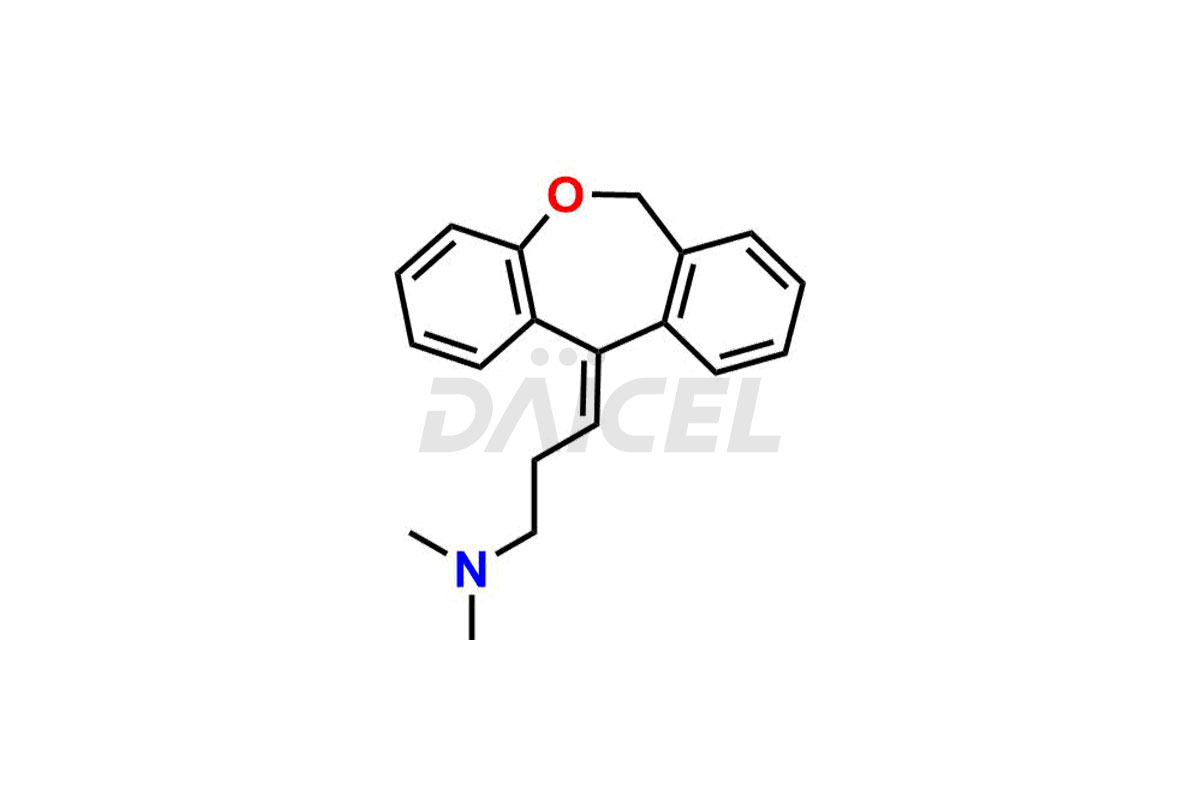
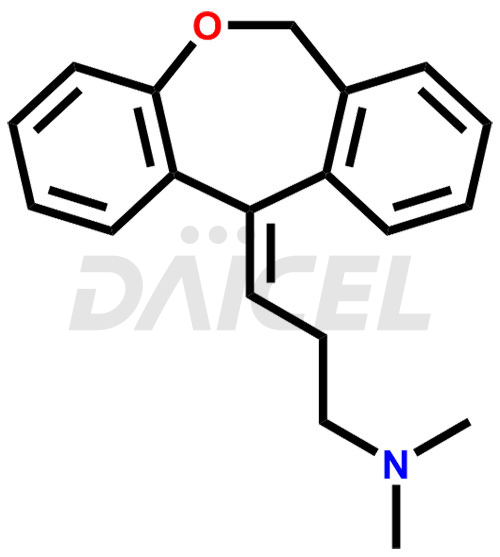
Doxepin [CAS: 1668-19-5], a dibenzoxepin derivative is a tricyclic antidepressant (TCA). It has antipruritic, antidepressant, and anxiolytic properties. It treats depression, anxiety, and insomnia.
Doxepin: Use and Commercial Availability
Doxepin, a member of the tricyclic antidepressant class, is prescribed for various conditions, including major depressive disorder, anxiety, insomnia, and skin pruritus management. The drug is available under brands such as Silenor, Sinequan, and Zonalon.
Doxepin Structure and Mechanism of Action 
The chemical name of Doxepin is 3-Dibenz[b,e]oxepin-11(6H)-ylidene-N,N-dimethyl-1-propanamine. Its chemical formula is C19H21NO, and its molecular weight is approximately 279.3 g/mol.
Doxepin binds with the histamine H1 receptor and acts as an antagonist.
Doxepin Impurities and Synthesis
The synthesis, analysis, and control of Doxepin impurities are critical aspects in ensuring the quality of the Doxepin drug. During the manufacturing process1, unintended substances or byproducts may arise as impurities. Rigorous analysis techniques, such as high-performance liquid chromatography (HPLC), help identify and quantify these impurities. Stringent control measures help limit the presence of Doxepin impurities, adhering to regulatory guidelines and maintaining the purity and safety of Doxepin.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Doxepin impurity standards, including E-Doxepin and Z-Doxepin. They generate from an analytical facility that complies with cGMP standards. The CoA gives a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We provide additional data like 13C-DEPT upon request. Daicel Pharma is synthesizing unknown Doxepin impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Process for the production of basic dibenzo-oxepin derivatives and their acid-addition salts, C. F. Boehringer & Soehne G.m.b.H., GB1004683A, September 15, 1965
- Wallace, Jack E.; Hamilton, Horace E.; Olivares, Rebecca; Harris, Steven C., Determination of doxepin by electron-capture gas chromatography, Journal of Analytical Toxicology, Volume: 2, Issue: 2, Pages: 44-9, 1978
Frequently Asked Questions
What control measures are in place for impurities in Doxepin?
Stringent control measures are implemented during the manufacturing process to minimize the formation of impurities in Doxepin. These include process optimization, purification techniques, and quality control checks.
Can Doxepin impurities impact the bioavailability or pharmacokinetics of the drug?
Depending on their nature and concentration, impurities in Doxepin can potentially affect the bioavailability and pharmacokinetics of the drug, which may influence its therapeutic efficacy and safety.
Which solvent helps in analyzing Doxepin impurities?
Acetonitrile is the solvent used for analyzing many impurities in Doxepin.
How should Doxepin impurities be stored in terms of temperature?
Doxepin impurities are stored at a controlled room temperature between 2-8 °C or according to the Certificate of Analysis (CoA) specifications.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.