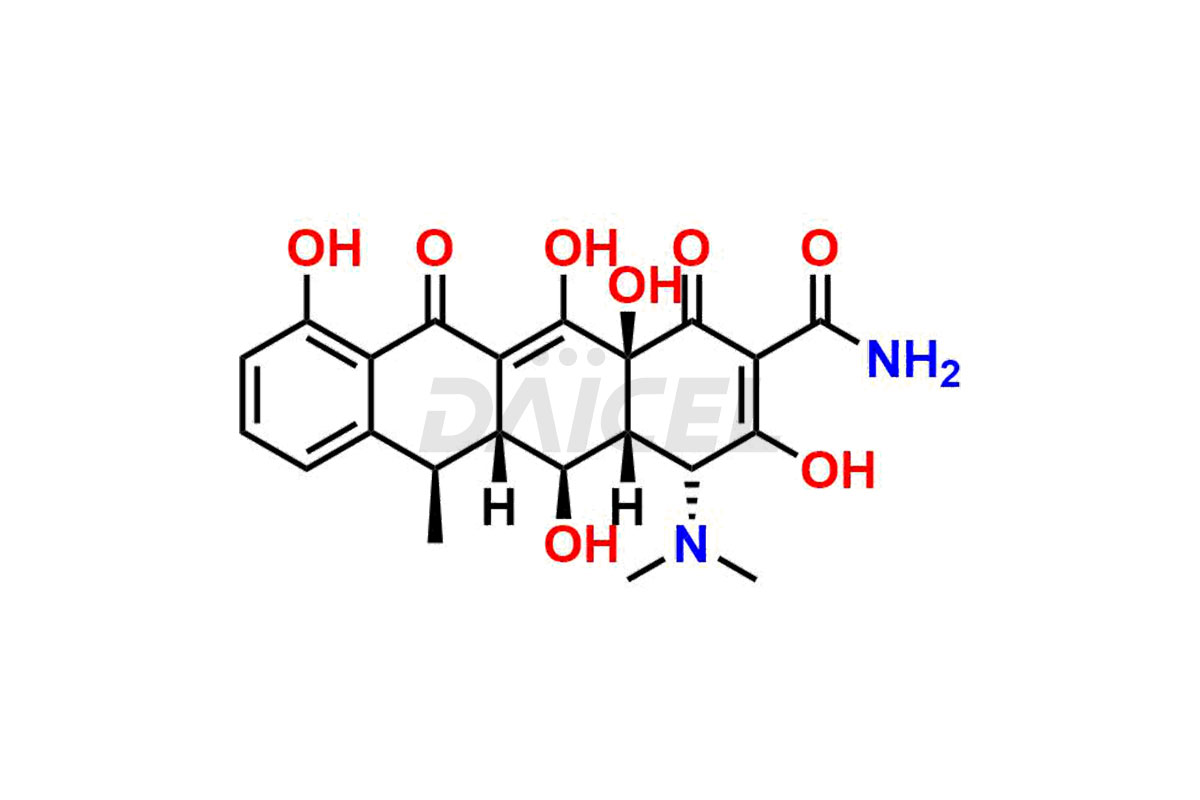
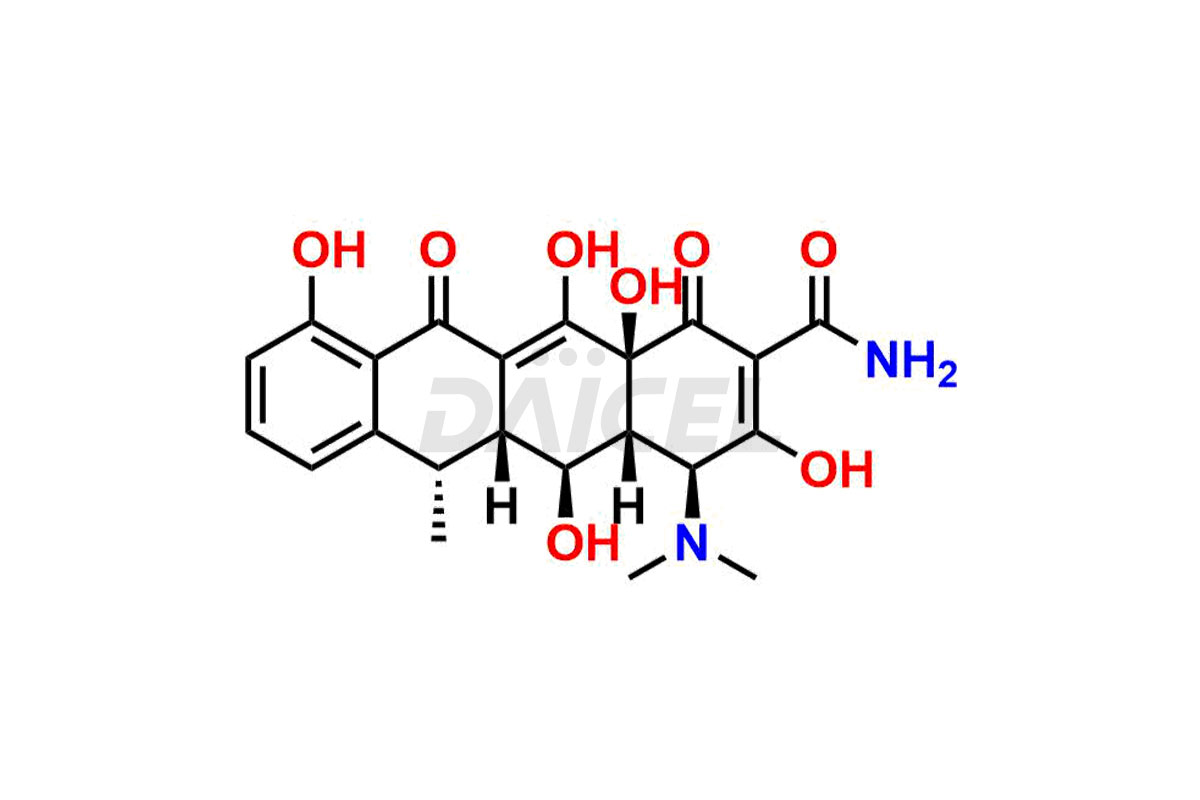
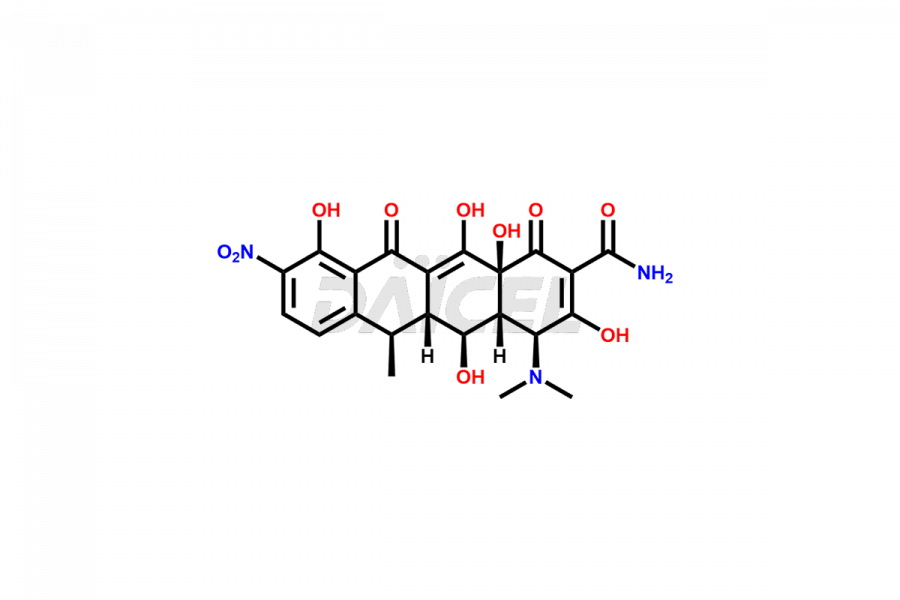
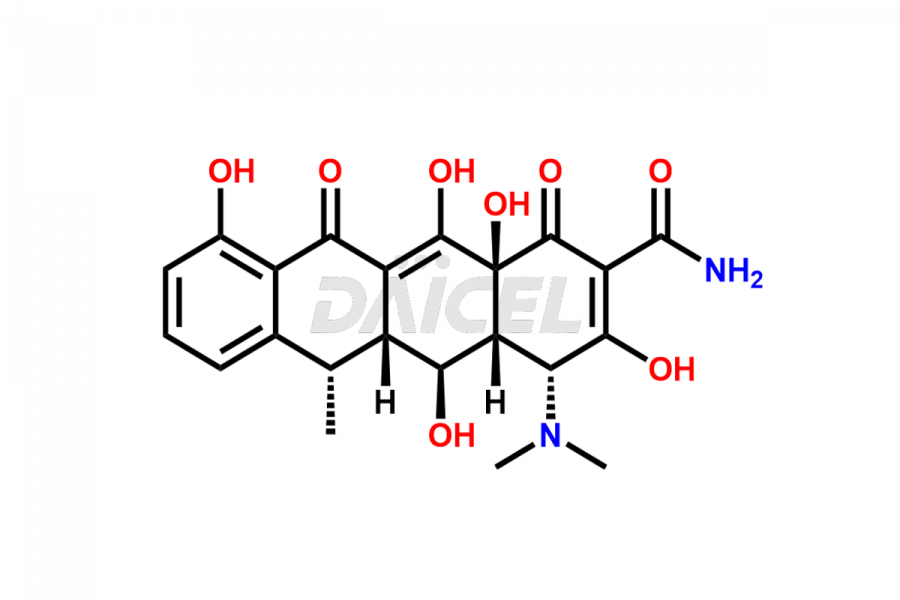
Doxycycline
General Information
Doxycycline Impurities and Doxycycline
Daicel Pharma synthesizes high-quality Doxycycline impurities, Doxycycline Impurity D, 4-Epidoxycycline, and 6-Epidoxycycline, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Doxycycline. Moreover, Daicel Pharma offers custom synthesis of Doxycycline impurities and delivers them globally.
Doxycycline [CAS: 564-25-0] is an antibiotic with broad-spectrum bacteriostatic properties, derived synthetically from a naturally occurring tetracycline known as oxytetracycline, produced by bacteria of the Streptomyces species. Doxycycline belongs to the tetracycline class of antibiotics.
Doxycycline: Use and Commercial Availability
Doxycycline is effective against a wide range of bacteria, including both gram-positive and gram-negative, aerobic and anaerobic bacteria, mycoplasma, and spirochetes. It has demonstrated particular effectiveness against several periodontal pathogens such as Porphyromonas gingivalis, Bacteroides forsythus, Prevotella intermedia, Actinobacillus actinomycetemcomitans, Campylobacter rectus, and Fusobacterium nucleatum. Doxycycline is available under several brand names, including Acticlate, Doryx, Doxy, Monodox, Oracea, Vibramycin, Xyrosa, and Zenavod, among others.
Doxycycline Structure and Mechanism of Action
The chemical name of Doxycycline is (4S,4aR,5S,5aR,6R,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide. Its chemical formula is C22H24N2O8 and its molecular weight is approximately 444.4 g/mol.
Doxycycline is an antimicrobial drug, that inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit.
Doxycycline Impurities and Synthesis
Doxycycline can contain impurities that may form during the synthesis1, storage, or transportation. These impurities include epimeric impurities, which form during the synthesis of Doxycycline. They can impact the drug’s stability, efficacy, and safety, and their levels must be monitored and controlled during manufacturing, storage, and transportation.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Doxycycline impurity standards, Doxycycline Impurity D, 4-Epidoxycycline, and 6-Epidoxycycline. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Doxycycline impurity or degradation product.
References
FAQ's
References
- 6-Deoxytetracycline and 6-deoxyoxytetracycline, American Cyanamid Co., GB845649A, August 24, 1960
- De Leenheer, Andre P.; Nelis, Hans J. C. F., Doxycycline determination in human serum and urine by high-performance liquid chromatography, Journal of Pharmaceutical Sciences, Volume: 68, Issue: 8, Pages: 999-1002, 1979
Frequently Asked Questions
What is the role of the Liquid Chromatographic (LC) Method in the analysis of Doxycycline impurities?
The LC method involves the separation of impurities present in Doxycycline by passing a mixture of the sample and a mobile phase through a chromatographic column. The separated impurities are detected and quantified using a detector. The LC method helps in identifying and quantifying organic and inorganic impurities. This method is selective, precise, and accurate for determining impurities in Doxycycline.
What are the most common impurities in Doxycycline?
The most common impurities in Doxycycline are related substances formed during manufacturing, such as 4-Epidoxycycline and 6-Epidoxycycline.
Which solvent helps in the analysis of Doxycycline impurities?
Methanol is a solvent for analyzing Doxycycline impurities.
What are the temperature conditions required to store Doxycycline impurities?
Doxycycline impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.