E & L Standards - Rubber Oligomers
General Information
Rubber oligomer impurities and Extractable & Leachable Standards
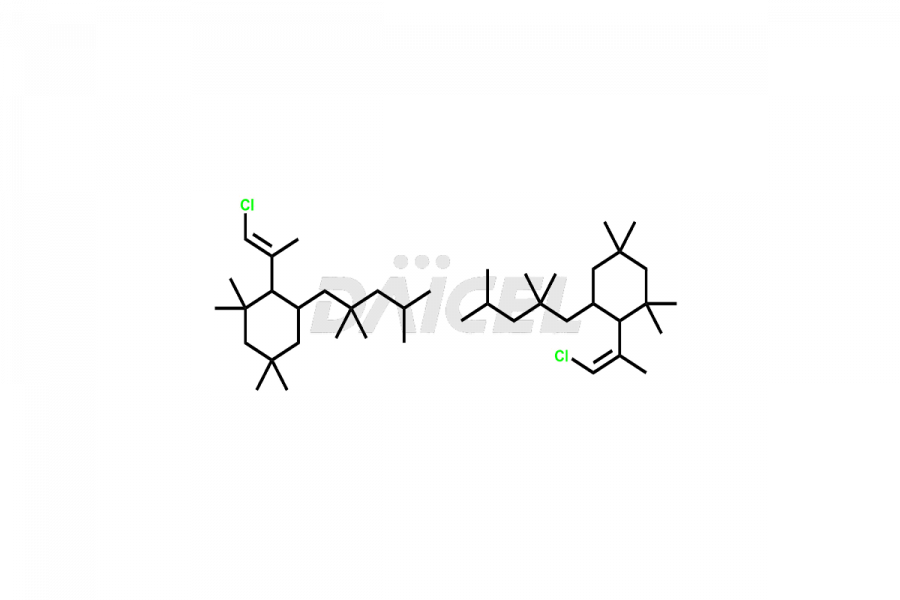
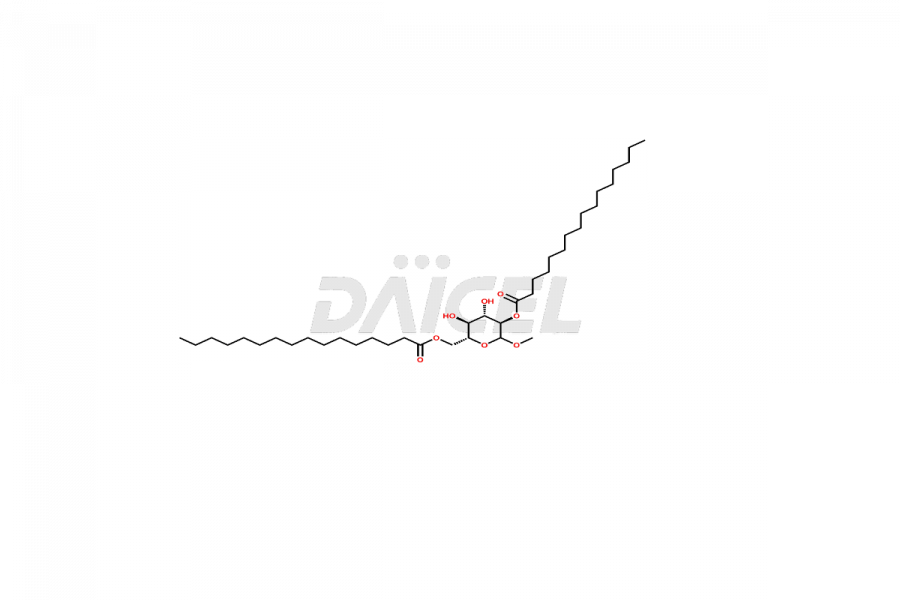
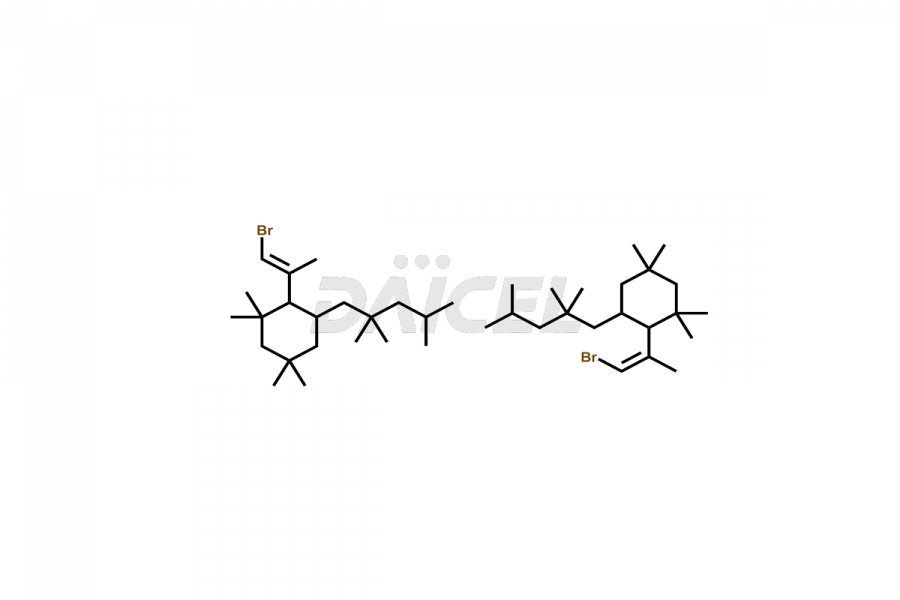
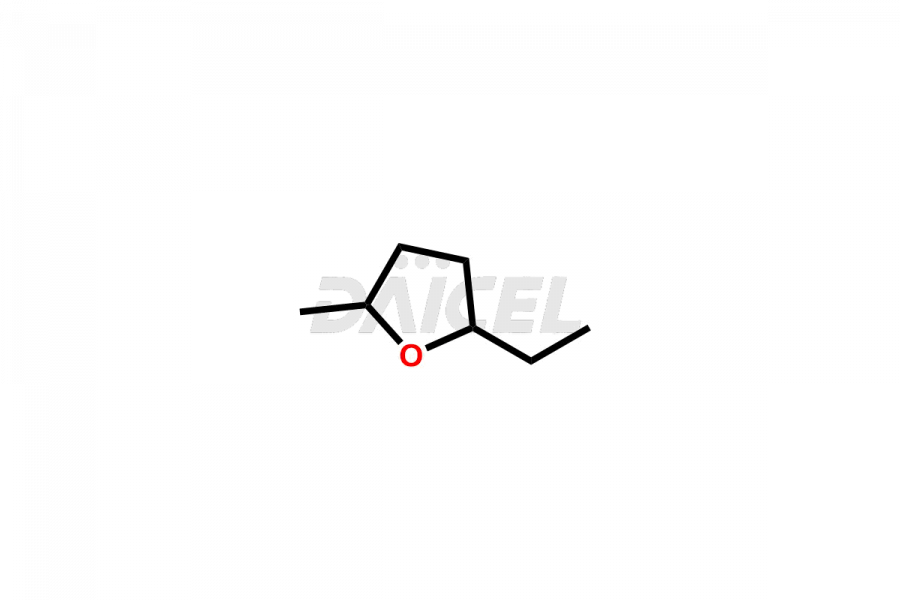
Daicel Pharma offers high-quality E&L Standards, Rubber Oligomers impurities, such as Rubber Oligomer-1, Rubber Oligomer 6, Rubber oligomer– 8, Rubber oligomer -9, Rubber Oligomer 10, 4-t-Butyl-2-(1-methyl-2-nitroethyl)cyclohexanone, 7,9-di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione, and Vinyl cinnamate. Understanding and controlling extractable and leachable is crucial for ensuring pharmaceutical safety, quality, and efficacy. Comprehensive studies and evaluations help mitigate risks associated with these contaminants, thereby protecting patient health and ensuring compliance with regulatory standards. Daicel Pharma specializes in the custom synthesis of Rubber Oligomers impurities and ensures their worldwide delivery.
Natural rubber undergoes fragmentation chemically or biologically, to isoprenoid oligomers. Rubber Oligomers form from rubber extracts. Rubber Oligomer impurities interact with peptides and proteins in container closure systems and cause drug toxicity. The interactions usually occur in pre-filled syringes. The impurities can affect product quality and immunogenicity.
Rubber Oligomers impurities: regulatory aspects
ICH Q3E guidelines for Extractables and Leachables (E&L) are under consideration by an Expert Working Group. Good Manufacturing Practices (GMP) regulations apply to all manufacturing stages, processing, and packaging.
Rubber oligomers composition
Rubber oligomers may consist of halogenated cyclic aliphatic hydrocarbons. C13H24 and C21H40 are the chemical formulas of common Rubber oligomers. NMR is used to elucidate the structures of rubber oligomers.
Rubber Oligomers Impurities and Synthesis
During the synthesis, storage, packing of drugs, Rubber Oligomer impurities may form that may affect the safety and efficacy of the drug. Drug manufacturers can control and monitor1 that Rubber Oligomer impurities so that they do not leach into drug products, affecting their safety and efficacy.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for E&L Standards, Rubber Oligomer impurities, which includes Rubber Oligomer-1, Rubber Oligomer 6, Rubber oligomer – 8, Rubber oligomer -9, Rubber Oligomer 10, 4-t-Butyl-2-(1-methyl-2-nitroethyl)cyclohexanone, 7,9-di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione, and Vinyl cinnamate. An issued CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data2 such as GC, 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Daicel Pharma can prepare any unidentified Rubber Oligomers impurity or degradation product. We also provide a complete characterization report on delivery
References
FAQ's
References
- Dennis Jenke, Identification, analysis and safety assessment of leachables and extractables, TrAC Trends in Analytical Chemistry, Volume 101, April 2018, Pages 56-65 (https://doi.org/10.1016/j.trac.2017.10.024)
- Richard Jähnke, Contamination of Injectable Powders by Volatile Hydrocarbons from Rubber Stoppers, Acta Pharmaceutica Technologica 36(3):139-148 (https://www.researchgate.net/publication/321197618_Contamination_of_Injectable_Powders_by_Volatile_Hydrocarbons_from_Rubber_Stoppers)
Frequently Asked Questions
What are the analytical methods to identify Rubber Oligomer impurities?
GC-MS, UHPLC-CAD, and headspace MS are the analytical methods to identify Rubber Oligomer impurities.
Why is it essential to remove Rubber Oligomer impurities from drug products?
Post-marketing product recalls are avoided by removing Rubber Oligomer impurities from drug products.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.