LOAD MORE
You're viewed 9 of 37 products
Daicel Pharma offers high-quality impurities for Edoxaban, an active pharmaceutical ingredient. These impurities, including EDB KSM1-RRR Isomer Impurity, Edoxaban 12 Dimer, Edoxaban 4-carboxylic acid, and more, play a vital role in assessing the purity, reliability, and safety of Edoxaban. Daicel Pharma also offers a customized synthesis of Edoxaban impurities to cater to client requirements, with worldwide delivery options available.
Edoxaban [CAS: 480449-70-5] is an oral, small-molecule inhibitor of coagulation factor Xa, exhibiting anticoagulant properties. It reduces the risk of venous thromboses, systemic embolization, and stroke in patients with atrial fibrillation. Additionally, it treats deep vein thrombosis and pulmonary embolism.
Edoxaban, available under Savaysa, is a direct oral anticoagulant (DOAC) that inhibits factor Xa (FXa). It treats deep venous thrombosis and pulmonary embolism. It reduces the risk of hypercoagulability-related illness, stroke, and systemic embolism (SE) in nonvalvular atrial fibrillation (NVAF) patients.
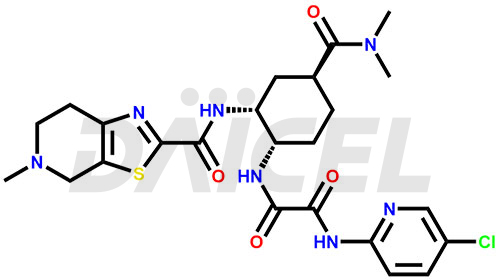
The chemical name of Edoxaban is N1-(5-Chloro-2-pyridinyl)-N2-[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]ethanediamide. Its chemical formula is C24H30ClN7O4S, and its molecular weight is approximately 548.06 g/mol.
Edoxaban is an FXa inhibitor that inhibits thrombin-induced platelet aggregation. It prevents prothrombinase activity and reduces thrombin formation.
The analysis and control of impurities in Edoxaban, a pharmaceutical compound, are essential to ensure its quality and safety. During the synthesis1, storage, or degradation of Edoxaban, various impurities may arise, which can impact its efficacy or pose risks to patients. The rigorous analysis identifies and quantifies these impurities, enabling their control within acceptable limits. Stringent control measures throughout the manufacturing process help minimize impurity levels and maintain the desired quality of Edoxaban. These measures include adhering to appropriate storage conditions, using robust manufacturing techniques, and conducting regular quality assessments to ensure the safety and effectiveness of the final product.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Edoxaban impurities, including EDB KSM1-RRR Isomer Impurity, Edoxaban 12 Dimer, Edoxaban 4-carboxylic acid, and more. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We provide additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Edoxaban impurities or degradation products, and labeled compounds, to evaluate the efficacy of generic Edoxaban. Also, Edoxaban-D6, a deuterium-labeled Edoxaban standard, is available for bio-analytical research, including BA/BE studies. Every delivery has a complete characterization report.
Yes, impurities in Edoxaban are closely monitored during clinical trials to assess their impact on safety, efficacy, and patient tolerability. Any significant impurities are thoroughly evaluated and controlled.
Certain impurities in Edoxaban may have the potential to interact with other medications. Drug-drug interaction studies help evaluate such possibilities and ensure safe co-administration.
Storage conditions can influence the formation of impurities in Edoxaban. Proper storage, including temperature control and protection from light and moisture, is essential to minimize impurity formation.
Edoxaban impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.