Eganelisib
General Information
Eganelisib Impurities and Eganelisib
Daicel Pharma offers superior-quality Eganelisib impurities, such as Eganelisib Enantiomer. It is vital for evaluating the quality, stability, and biological safety of Eganelisib. In addition, Daicel Pharma specializes in the custom synthesis of Eganelisib impurities and ensures their worldwide delivery.
Eganelisib [CAS: 1693758-51-8] is under pre-clinical studies for treating patients with tumors. IPI-549, or Eganelisib, is a first-in-class PI3Kγ inhibitor. Phosphoinositide-3-kinase (PI3K)-γ inhibitors target PI3K isoforms during cancer therapy. The inhibitors have more than 150-fold selectivity over other protein kinases. Eganelisib is used alone or in combination with PD-1/PD-L1 inhibitors and acts against tumors.
Eganelisib: Use and Commercial Availability
Eganelisib is administered orally to patients with advanced solid tumors. Its antitumor activity in cancer patients is under clinical investigation. Studies are on to check its effects in monotherapy or combination with PD-1 inhibitor nivolumab1.
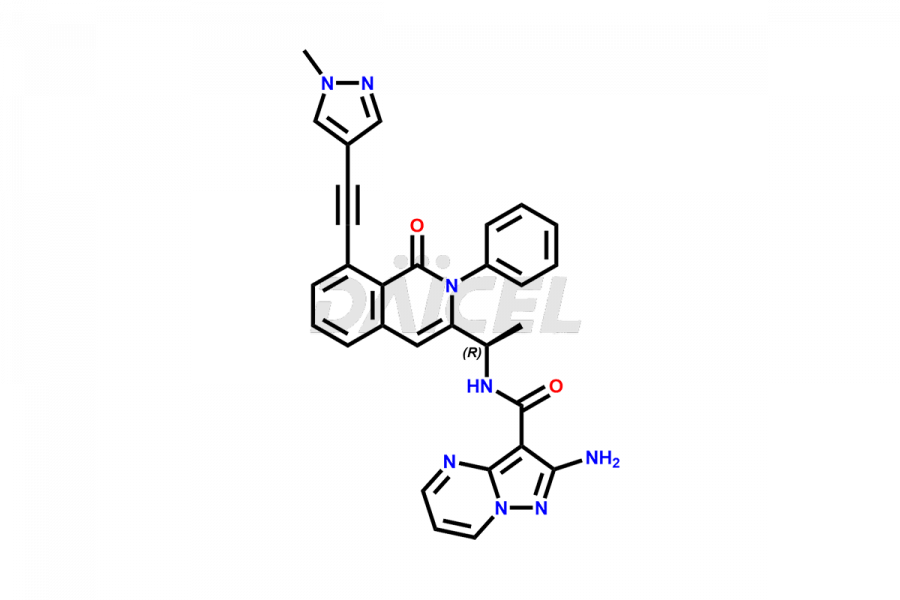
Eganelisib Structure and Mechanism of Action

The chemical name of Eganelisib is 2-Amino-N-[(1S)-1-[1,2-dihydro-8-[2-(1-methyl-1H-pyrazol-4-yl)ethynyl]-1-oxo-2-phenyl-3-isoquinolinyl]ethyl]pyrazolo[1,5-a]pyrimidine-3-carboxamide. The chemical formula for Eganelisib is C30H24N8O2 and its molecular weight is approximately 528.57 g/mol.
Eganelisib can inhibit tumor growth. However, the exact mechanism of action of Eganelisib is not known.
Eganelisib Impurities and Synthesis
During the synthesis of Eganelisib 2, impurities may form that may affect the safety and efficacy of the drug. These impurities are generated during the synthesis, storage, or degradation of Eganelisib. Eganelisib impurities need control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Eganelisib impurities, which includes Eganelisib Enantiomer. A CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Eganelisib impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Eganelisib for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
- David S. Hong, Michael Postow, Bartosz Chmielowski,Ryan Sullivan, Amita Patnaik, Ezra E.W. Cohen,Geoffrey Shapiro, Conor Steuer, Martin Gutierrez, Heather Yeckes-Rodin, Robert Ilaria, Jr, Brenda O'Connell, Joanna Peng, Guangbin Peng, Nora Zizlsperger, Anthony Tolcher, Jedd D. Wolchok, Clin Cancer Res (2023) 29 (12): 2210–2219
- Castro, Alfredo C.; Evans, Catherine A.; Janardanannair, Somarajannair; Lescarbeau, Andre; Liu, Tao; Tremblay, Martin R., Heterocyclic compounds and uses thereof, WO2015051244A1, April 9, 2015, Infinity Pharmaceuticals, Inc., United States
Frequently Asked Questions
What are the kinds of impurities in drug substances?
What are the analytical methods to identify unknown impurities?
What is an enantiomeric impurity?
How do you minimize impurity risks during drug manufacturing?
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.