LOAD MORE
You're viewed 9 of 20 products
Daicel Pharma offers high-quality impurities for Elagolix, an active pharmaceutical ingredient. These impurities, including Desbutyrate Elagolix, Elagolix Desfluoro Impurity 1, Elagolix Enantiomer Impurity, Elagolix Impurity B, Elagolix Impurity-C, Elagolix Impurity D, Elagolix Sodium Impurity-E, and more, play a vital role in assessing the purity, reliability, and safety of Elagolix. Daicel Pharma also offers a customized synthesis of Elagolix impurities to cater to client requirements, with worldwide delivery options available.
Elagolix [CAS: 834153-87-6], an orally bioavailable, second-generation compound, is a selective antagonist of the gonadotropin-releasing hormone (GnRH; LHRH) receptor. It is a small molecule, a non-peptide-based compound that inhibits hormone production. This medication treats moderate to severe pain associated with endometriosis.
Elagolix, available under Orilissa, is an oral GnRH antagonist that effectively suppresses pituitary-ovarian hormones in a dose-dependent manner. It can partially control ovarian estrogen production at lower doses. Elagolix effectively inhibits the production of gonadotropins and estrogen. It also reduces average endometrial thickness without affecting the serum anti-Müllerian hormone (AMH) level.
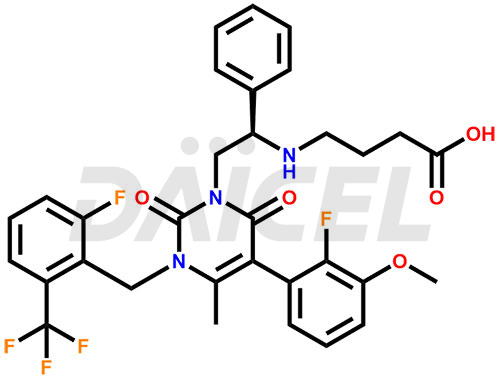
The chemical name of Elagolix is 4-[[(1R)-2-[5-(2-Fluoro-3-methoxyphenyl)-3-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-3,6-dihydro-4-methyl-2,6-dioxo-1(2H)-pyrimidinyl]-1-phenylethyl]amino]butanoic acid. Its chemical formula is C32H30F5N3O5, and its molecular weight is approximately 631.6 g/mol.
Elagolix binds to GnRH receptors in the pituitary gland and inhibits endogenous GnRH signaling. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are controlled. It leads to a decrease in blood concentration of progesterone and estradiol.
The analysis and control of impurities in Elagolix, a pharmaceutical compound, are vital to ensure its quality and safety. During the synthesis1,2, storage, or degradation of Elagolix, various impurities may arise that can impact its efficacy or pose potential risks. Th extensive analysis is conducted to identify and quantify these impurities, enabling their control within acceptable limits. Strict control measures throughout the manufacturing process help minimize impurity levels and maintain the desired quality of Elagolix. It includes adherence to appropriate storage conditions, stringent manufacturing practices, and regular quality assessments to ensure the safety and effectiveness of the final product.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Elagolix impurity standards, including Desbutyrate Elagolix, Elagolix Desfluoro Impurity 1, Elagolix Enantiomer Impurity, Elagolix Impurity B, Elagolix Impurity-C, Elagolix Impurity D, Elagolix Sodium Impurity-E, and more. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis3. We provide additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Elagolix impurities or degradation products. Elagolix-D6, a deuterium-labeled Elagolix standard, is available for bio-analytical research, including BA/BE studies. Every delivery has a complete characterization report.
Some impurities in Elagolix can affect its bioavailability, which refers to the drug's rate and extent of absorption. Careful control of impurity levels ensures consistent and predictable drug absorption.
Regulatory agencies such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have specific guidelines and requirements for controlling impurities in pharmaceutical products, including Elagolix.
Impurities in Elagolix can potentially impact its therapeutic efficacy by altering the drug's pharmacological activity. Therefore, strict control measures are in place to ensure they are within acceptable limits to maintain the drug's desired therapeutic effect.
Elagolix impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.