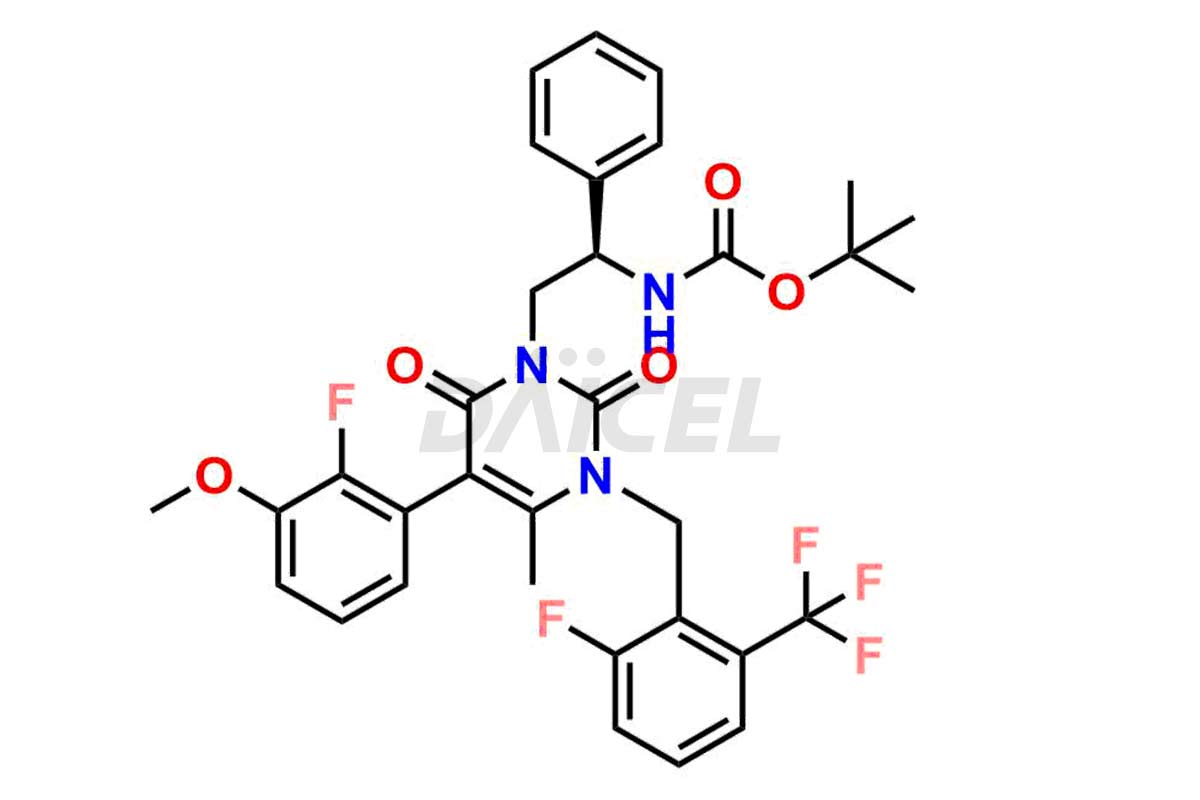
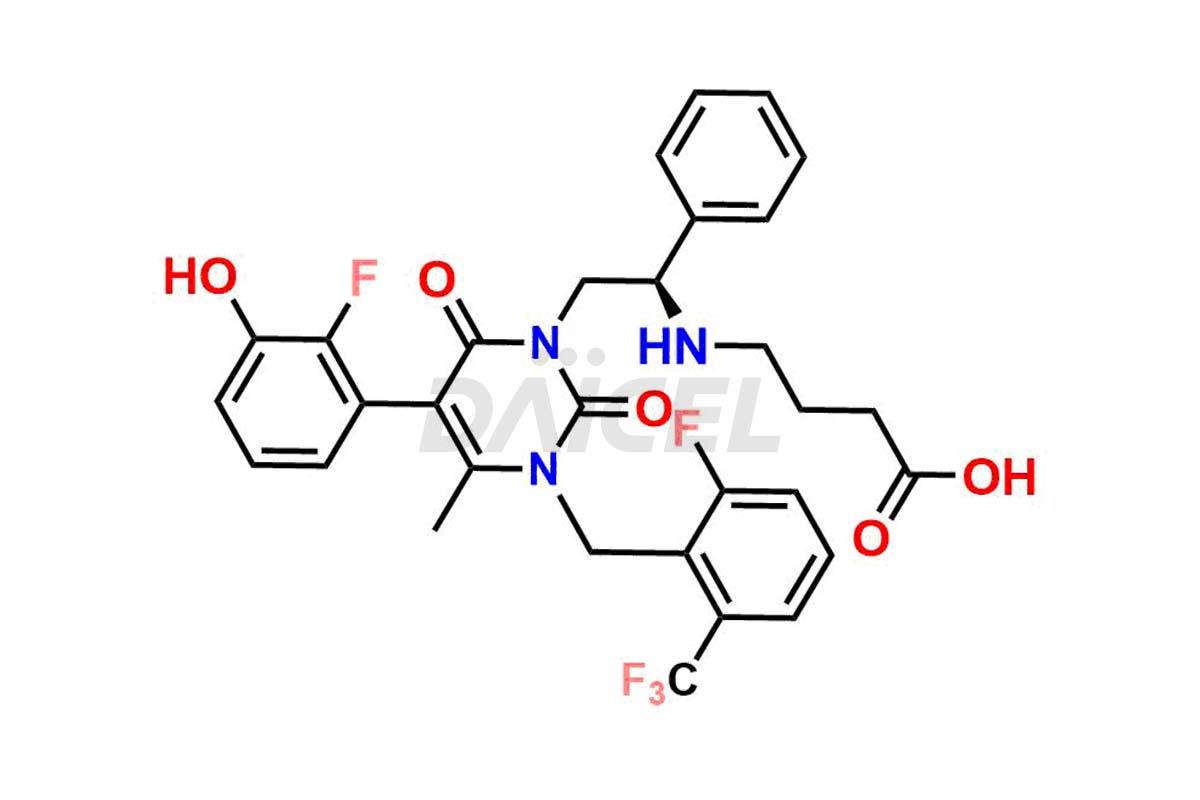
Elagolix
References
- Chen, Chen; Wu, Dongpei; Guo, Zhiqiang; Xie, Qiu; Reinhart, Greg J.; Madan, Ajay; Wen, Jenny; Chen, Takung; Huang, Charles Q.; Chen, Mi; et al, Discovery of Sodium R-(+)-4-{2-[5-(2-Fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (Elagolix), a Potent and Orally Available Nonpeptide Antagonist of the Human Gonadotropin-Releasing Hormone Receptor, Journal of Medicinal Chemistry, Volume: 51, Issue: 23, Pages: 7478-7485, 2008
- Gallagher, Donald; Treiber, Laszlo; Hughes, Robert; Campopiano, Onorato; Wang, Peng; Zhao, Yuxin; Chou, Shine; Ouellette, Michael; Hettinger, Donald, Processes for the preparation of uracil derivatives, Neurocrine Biosciences, Inc., United States, US8765948B2, July 1, 2014
- Bommi, Sivaganesh; Jayanty, Subbalakshmi; Tirumalaraju, Satyanarayana Raju; Kola, Suresh; Kallam, Venkata Siva Rama Krishna Reddy, Evolution of Stability-Indicating Method in the Quantification of Related Substances and Degradation Products of Elagolix Sodium: Quality by Design-Driven Approach Utilizing Ultra-high Performance Liquid Chromatography, Chromatographia, Volume: 86,Issue: 1,Pages: 31-42, 2023
Frequently Asked Questions
Can Elagolix impurities impact its bioavailability?
Some impurities in Elagolix can affect its bioavailability, which refers to the drug's rate and extent of absorption. Careful control of impurity levels ensures consistent and predictable drug absorption.
Are there any specific regulatory guidelines for controlling Elagolix impurities?
Regulatory agencies such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have specific guidelines and requirements for controlling impurities in pharmaceutical products, including Elagolix.
Can Elagolix impurities affect its therapeutic efficacy?
Impurities in Elagolix can potentially impact its therapeutic efficacy by altering the drug's pharmacological activity. Therefore, strict control measures are in place to ensure they are within acceptable limits to maintain the drug's desired therapeutic effect.
What are the temperature conditions required to store Elagolix impurities?
Elagolix impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.