Etonogestrel
General Information
Etonogestrel Impurities and Etonogestrel
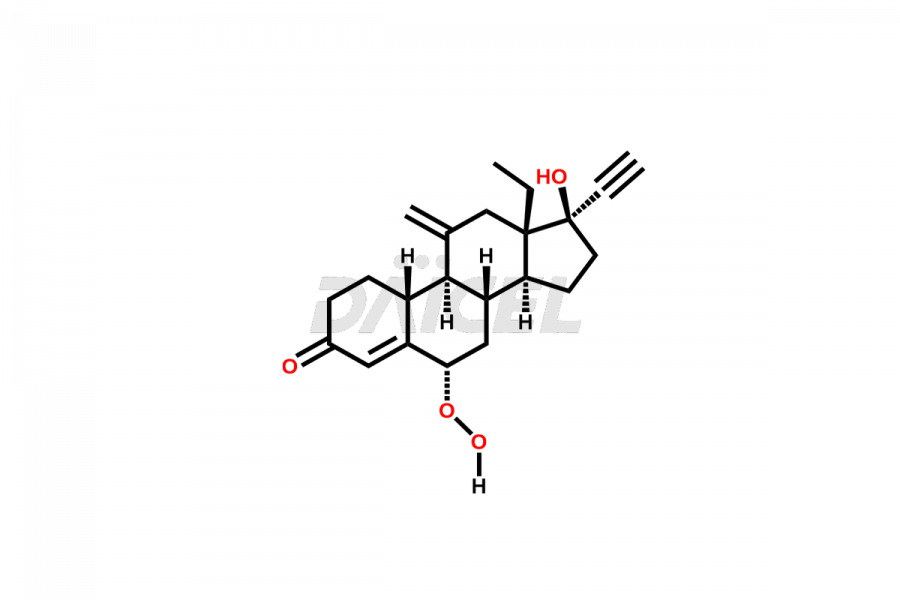
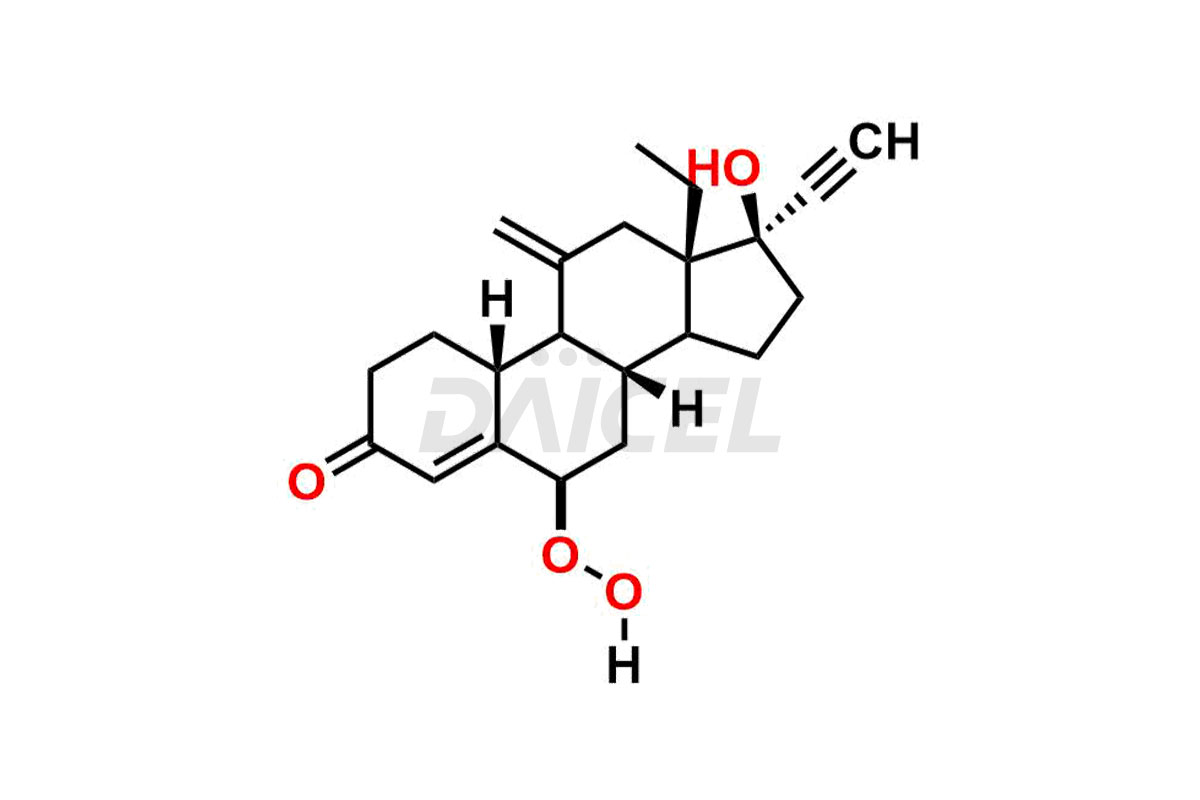
Daicel Pharma offers high-quality impurities for Etonogestrel, an active pharmaceutical ingredient. These impurities, including 6α-hydroxy peroxide etonogestrel and 6β-hydroxy peroxide etonogestrel, play a vital role in assessing the purity, reliability, and safety of Etonogestrel. Daicel Pharma also offers a customized synthesis of Etonogestrel impurities to cater to client requirements, with worldwide delivery options available.
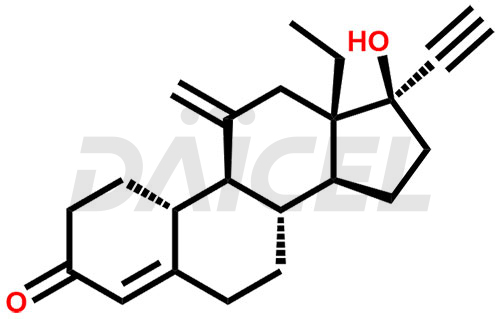
Etonogestrel [CAS: 54048-10-1] is a synthetic progestin derived from progesterone, a natural hormone in females. It is a 17-beta-hydroxy steroid and a 3-oxo-Delta (4) steroid. Etonogestrel acts as a contraceptive drug for females.
Etonogestrel: Use and Commercial Availability
Etonogestrel is available under various brand names, including Implanon and Nexplanon. It is administered through subdermal implants, offering long-acting reversible contraception. These implants are effective for up to 5 years in postpartum women, including breastfeeding women. Etonogestrel is a chief component of these contraceptive implants, providing a reliable method of preventing pregnancy.
Etonogestrel Structure and Mechanism of Action 
The chemical name of Etonogestrel is (17α)-13-Ethyl-17-hydroxy-11-methylene-18,19-dinorpregn-4-en-20-yn-3-one. Its chemical formula is C22H28O2, and its molecular weight is approximately 324.5 g/mol.
Etonogestrel suppresses ovulation and increases cervical mucus viscosity and endometrium alterations.
Etonogestrel Impurities and Synthesis
Impurities in Etonogestrel are unintended substances or contaminants that can be present in the medication. These impurities can occur during the manufacturing1, synthesis, or storage processes. They may include related compounds, degradation products, residual solvents, or other impurities introduced during production. The impurities can affect the purity, stability, and overall quality of Etonogestrel. Strict regulatory guidelines and quality control measures help monitor and limit the levels of impurities to ensure the safety and efficacy of Etonogestrel medications.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Etonogestrel impurity standards, including 6α-hydroxy peroxide etonogestrel and 6β-Hydroxy peroxide etonogestrel. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Etonogestrel impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Winterfeldt, Ekkehard; Tilstamm, Ulf; Hofmeister, Helmut; Laurent, Henry, Method For The Preparation Of 3-Desoxy-4-Ene Steroids, Schering A.-G., Germany, EP440752B1, March 8, 1995
- Thomas, Tiffany; Petrie, Kelsey; Shim, Joonho; Abildskov, Kirsten M.; Westhoff, Carolyn L.; Cremers, Serge, A UPLC-MS/MS Method for Therapeutic Drug Monitoring of Etonogestrel, Therapeutic Drug Monitoring, Volume: 35, Issue: 6, Pages: 844-848, 2013
Frequently Asked Questions
How are Etonogestrel impurities monitored during the manufacturing process?
In-process controls and regular quality control testing are implemented during the manufacturing process of Etonogestrel to monitor impurity levels and ensure compliance with specifications.
Can Etonogestrel impurities be removed through purification processes?
Purification steps during the manufacturing process of Etonogestrel help remove or reduce impurities to acceptable levels.
What solvent helps in analyzing Etonogestrel impurities?
Methanol is the solvent used for analyzing most of the impurities in Etonogestrel.
How should Etonogestrel impurities be stored in terms of temperature?
Etonogestrel impurities are stored at a controlled room temperature, 2-8 °C. Etonogestrel impurities such as 6β-Hydroxy peroxide Etonogestrel are stored below -10 °C or as specified in the COA.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.