Etravirine
General Information
Etravirine Impurities and Etravirine
Daicel Pharma offers the best Etravirine impurities, such as Etravirine Butanamide impurity. It is vital for evaluating the quality, stability, and biological safety of Etravirine. In addition, Daicel Pharma specializes in the custom synthesis of Etravirine impurities and ensures their worldwide delivery.
Etravirine [CAS: 269055-15-4], a diarylpyrimidine (DAPY), along with other antiretroviral agents, treats patients with human immunodeficiency virus type 1 (HIV-1) infection. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI). It is a second-generation NNRTI that has antiviral activity against HIV-1 strains. Further, it was approved by the US FDA.
Etravirine: Use and Commercial Availability
Etravirine is an orally administered drug for treating HIV infection in adults with prior exposure to HIV medicines. In combination with other NNRTIs and antiretroviral agents, Etravirine treats HIV infection and the acquired immunodeficiency syndrome (AIDS). It is effective against NNRTI resistance-associated mutations. It is available under the name Intelence.
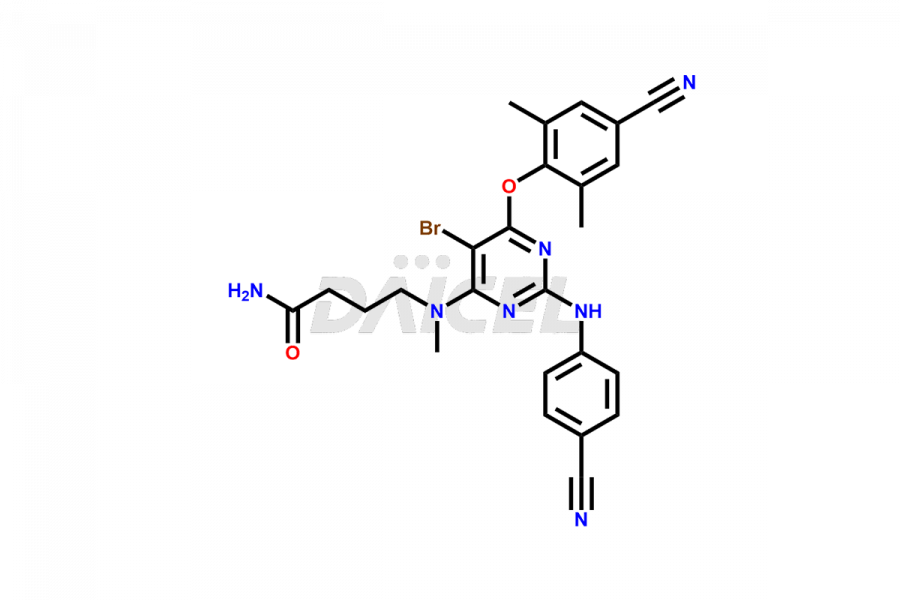
Etravirine Structure and Mechanism of Action


The chemical name of Etravirine is 4-[[6-Amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzonitrile. The chemical formula for Etravirine is C20H15BrN6O, and its molecular weight is approximately 435.28g/mol.
Etravirine binds to reverse transcriptase (RT) directly. It disrupts the enzyme’s catalytic site and inhibits RNA-dependent and DNA-dependent DNA polymerase activities.
Etravirine Impurities and Synthesis
Impurities may form during the synthesis of Etravirine 1 that affect the safety, efficacy, and shelf-life. These impurities form during the synthesis, storage, or degradation of Etravirine. It is necessary to control and monitor the impurities of Etravirine to improve the safety, efficacy, and storage of the drug.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Etravirine impurities, which includes Etravirine Butanamide impurity. A CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS2, and HPLC purity. We give additional data like 13C-DEPT and CHN on request. Daicel Pharma can prepare any unidentified Etravirine impurity or degradation products under customs synthesis. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Etravirine for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
- De Corte, Bart; De Jonge, Marc Rene; Heeres, Jan; Ho, Chih Yung; Janssen, Paul Adriaan Jan; Kavash, Robert W.; Koymans, Lucien Maria Henricus; Kukla, Michael Joseph; Ludovici, Donald William; Van Aken, Koen Jeanne Alfons, HIV replication inhibiting pyrimidines, WO0027825A1, May 18, 2000, Janssen Pharmaceutica N.V., Belgium
- ter Heine, R.; Rosing, H.; van Gorp, E. C. M.; Mulder, J. W.; Beijnen, J. H.; Huitema, A. D. R., Quantification of etravirine (TMC125) in plasma, dried blood spots and peripheral blood mononuclear cell lysate by liquid chromatography tandem mass spectrometry, Journal of Pharmaceutical and Biomedical Analysis, Volume: 49, Issue: 2, Pages: 393-400, 2009
Frequently Asked Questions
What are organic and inorganic impurities?
What happens to drug products detected with impurities?
What are the conditions that cause Etravirine degradation?
What are the analytical methods for the quantification of Etravirine impurities?
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.